CHXI04:CHEMICAL BONDING AND MOLECULAR STRUCTURE
313974
Match Column-I with Column-II.
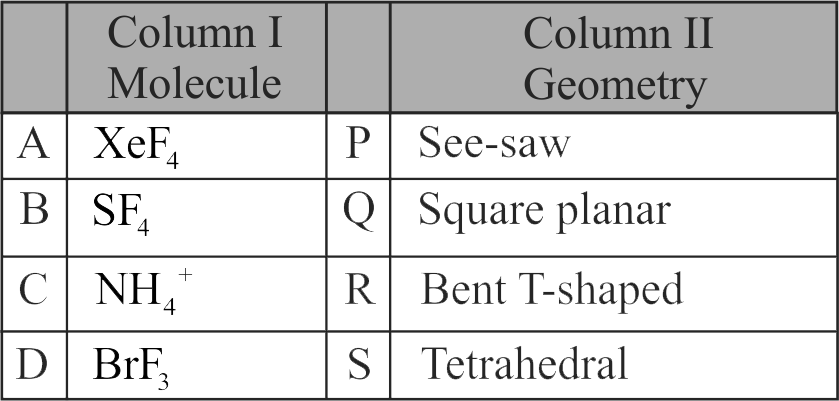
Choose the correct answer from the options given below:
Column I
Column II
1 A - S, B - P, C - Q , D - R
2 A - S, B - R, C - Q , D - P
3 A - Q , B - P, C - S, D - R
4 A - Q , B - P, C - R, D - S
Explanation:
hybridization - 2 lone pairs
– square planar.
hybridization -1 lone pair
– see - saw
hybridization – no lone pairs – tetrahedral
hybridization – 2 lone pairs – bent – T – shape.