313970
Assertion :
\(\mathrm{ClF}_{3}\) molecule is not isoelectronic with \(\mathrm{SF}_{4}\).
Reason :
Five electron pairs present in \(\mathrm{CIF}_{3}\) in total consist of two lps and three \({\text{bps}}\), and its geometry is \(\mathrm{T}\) - shaped.
313972
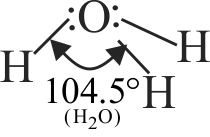
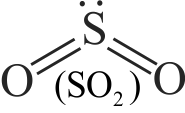
Statement A :
The structure of \({{\rm{H}}_{\rm{2}}}{\rm{O}}\) is similar to \({\rm{S}}{{\rm{O}}_{\rm{2}}}\).
Statement B :
In both molecules \(\left( {{{\rm{H}}_{\rm{2}}}{\rm{O}}\,\,{\rm{and}}\,\,{\rm{S}}{{\rm{O}}_{\rm{2}}}} \right){\rm{,}}\) the central atom has one lone pair of electrons.
313970
Assertion :
\(\mathrm{ClF}_{3}\) molecule is not isoelectronic with \(\mathrm{SF}_{4}\).
Reason :
Five electron pairs present in \(\mathrm{CIF}_{3}\) in total consist of two lps and three \({\text{bps}}\), and its geometry is \(\mathrm{T}\) - shaped.
313972
Statement A :
The structure of \({{\rm{H}}_{\rm{2}}}{\rm{O}}\) is similar to \({\rm{S}}{{\rm{O}}_{\rm{2}}}\).
Statement B :
In both molecules \(\left( {{{\rm{H}}_{\rm{2}}}{\rm{O}}\,\,{\rm{and}}\,\,{\rm{S}}{{\rm{O}}_{\rm{2}}}} \right){\rm{,}}\) the central atom has one lone pair of electrons.
313970
Assertion :
\(\mathrm{ClF}_{3}\) molecule is not isoelectronic with \(\mathrm{SF}_{4}\).
Reason :
Five electron pairs present in \(\mathrm{CIF}_{3}\) in total consist of two lps and three \({\text{bps}}\), and its geometry is \(\mathrm{T}\) - shaped.
313972
Statement A :
The structure of \({{\rm{H}}_{\rm{2}}}{\rm{O}}\) is similar to \({\rm{S}}{{\rm{O}}_{\rm{2}}}\).
Statement B :
In both molecules \(\left( {{{\rm{H}}_{\rm{2}}}{\rm{O}}\,\,{\rm{and}}\,\,{\rm{S}}{{\rm{O}}_{\rm{2}}}} \right){\rm{,}}\) the central atom has one lone pair of electrons.
313970
Assertion :
\(\mathrm{ClF}_{3}\) molecule is not isoelectronic with \(\mathrm{SF}_{4}\).
Reason :
Five electron pairs present in \(\mathrm{CIF}_{3}\) in total consist of two lps and three \({\text{bps}}\), and its geometry is \(\mathrm{T}\) - shaped.
313972
Statement A :
The structure of \({{\rm{H}}_{\rm{2}}}{\rm{O}}\) is similar to \({\rm{S}}{{\rm{O}}_{\rm{2}}}\).
Statement B :
In both molecules \(\left( {{{\rm{H}}_{\rm{2}}}{\rm{O}}\,\,{\rm{and}}\,\,{\rm{S}}{{\rm{O}}_{\rm{2}}}} \right){\rm{,}}\) the central atom has one lone pair of electrons.