313932
Match the Column I (Hybridisation) with Column II (Orientation in space).
| Column I | Column II |
|---|---|
| A. \({\rm{s}}{{\rm{p}}^{\rm{3}}}\) | P. Trigonal bipyramidal |
| B. \({\rm{ds}}{{\rm{p}}^{\rm{2}}}\) | Q. Octahedral |
| C. \({\rm{s}}{{\rm{p}}^{\rm{3}}}{\rm{d}}\) | R. Tetrahedral |
| D. \({\rm{s}}{{\rm{p}}^{\rm{3}}}{{\rm{d}}^{\rm{2}}}\) | S. Square planar |
314000
Match List-I with List-II
Choose the correct answer from the options given below
| Column I | Column II |
|---|---|
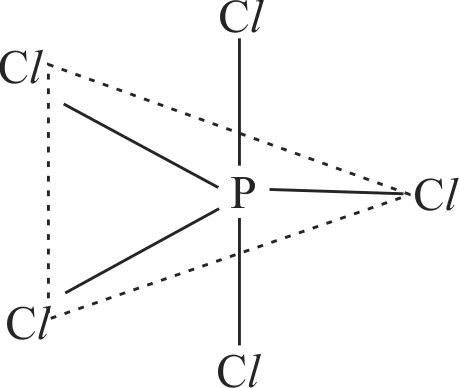
| A. \({\rm{PC}}{{\rm{l}}_{\rm{5}}}\) | P. Square pyramidal |
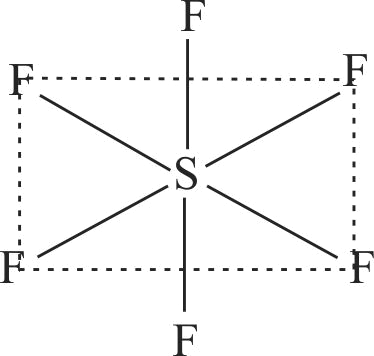
| B. \({\rm{S}}{{\rm{F}}_{\rm{6}}}\) | Q. Trigonal planar |
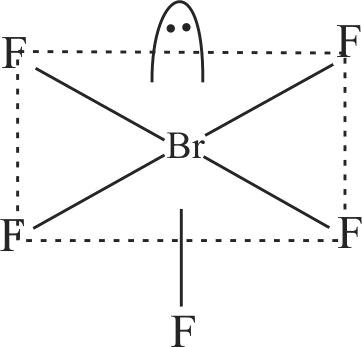
| C. \({\rm{Br}}{{\rm{F}}_{\rm{5}}}\) | R. Octahedral |
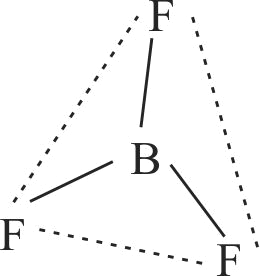
| D. \({\rm{B}}{{\rm{F}}_{\rm{3}}}\) | S. Trigonal bipyramidal |
313932
Match the Column I (Hybridisation) with Column II (Orientation in space).
| Column I | Column II |
|---|---|
| A. \({\rm{s}}{{\rm{p}}^{\rm{3}}}\) | P. Trigonal bipyramidal |
| B. \({\rm{ds}}{{\rm{p}}^{\rm{2}}}\) | Q. Octahedral |
| C. \({\rm{s}}{{\rm{p}}^{\rm{3}}}{\rm{d}}\) | R. Tetrahedral |
| D. \({\rm{s}}{{\rm{p}}^{\rm{3}}}{{\rm{d}}^{\rm{2}}}\) | S. Square planar |
314000
Match List-I with List-II
Choose the correct answer from the options given below
| Column I | Column II |
|---|---|
| A. \({\rm{PC}}{{\rm{l}}_{\rm{5}}}\) | P. Square pyramidal |
| B. \({\rm{S}}{{\rm{F}}_{\rm{6}}}\) | Q. Trigonal planar |
| C. \({\rm{Br}}{{\rm{F}}_{\rm{5}}}\) | R. Octahedral |
| D. \({\rm{B}}{{\rm{F}}_{\rm{3}}}\) | S. Trigonal bipyramidal |
313932
Match the Column I (Hybridisation) with Column II (Orientation in space).
| Column I | Column II |
|---|---|
| A. \({\rm{s}}{{\rm{p}}^{\rm{3}}}\) | P. Trigonal bipyramidal |
| B. \({\rm{ds}}{{\rm{p}}^{\rm{2}}}\) | Q. Octahedral |
| C. \({\rm{s}}{{\rm{p}}^{\rm{3}}}{\rm{d}}\) | R. Tetrahedral |
| D. \({\rm{s}}{{\rm{p}}^{\rm{3}}}{{\rm{d}}^{\rm{2}}}\) | S. Square planar |
314000
Match List-I with List-II
Choose the correct answer from the options given below
| Column I | Column II |
|---|---|
| A. \({\rm{PC}}{{\rm{l}}_{\rm{5}}}\) | P. Square pyramidal |
| B. \({\rm{S}}{{\rm{F}}_{\rm{6}}}\) | Q. Trigonal planar |
| C. \({\rm{Br}}{{\rm{F}}_{\rm{5}}}\) | R. Octahedral |
| D. \({\rm{B}}{{\rm{F}}_{\rm{3}}}\) | S. Trigonal bipyramidal |
313932
Match the Column I (Hybridisation) with Column II (Orientation in space).
| Column I | Column II |
|---|---|
| A. \({\rm{s}}{{\rm{p}}^{\rm{3}}}\) | P. Trigonal bipyramidal |
| B. \({\rm{ds}}{{\rm{p}}^{\rm{2}}}\) | Q. Octahedral |
| C. \({\rm{s}}{{\rm{p}}^{\rm{3}}}{\rm{d}}\) | R. Tetrahedral |
| D. \({\rm{s}}{{\rm{p}}^{\rm{3}}}{{\rm{d}}^{\rm{2}}}\) | S. Square planar |
314000
Match List-I with List-II
Choose the correct answer from the options given below
| Column I | Column II |
|---|---|
| A. \({\rm{PC}}{{\rm{l}}_{\rm{5}}}\) | P. Square pyramidal |
| B. \({\rm{S}}{{\rm{F}}_{\rm{6}}}\) | Q. Trigonal planar |
| C. \({\rm{Br}}{{\rm{F}}_{\rm{5}}}\) | R. Octahedral |
| D. \({\rm{B}}{{\rm{F}}_{\rm{3}}}\) | S. Trigonal bipyramidal |
313932
Match the Column I (Hybridisation) with Column II (Orientation in space).
| Column I | Column II |
|---|---|
| A. \({\rm{s}}{{\rm{p}}^{\rm{3}}}\) | P. Trigonal bipyramidal |
| B. \({\rm{ds}}{{\rm{p}}^{\rm{2}}}\) | Q. Octahedral |
| C. \({\rm{s}}{{\rm{p}}^{\rm{3}}}{\rm{d}}\) | R. Tetrahedral |
| D. \({\rm{s}}{{\rm{p}}^{\rm{3}}}{{\rm{d}}^{\rm{2}}}\) | S. Square planar |
314000
Match List-I with List-II
Choose the correct answer from the options given below
| Column I | Column II |
|---|---|
| A. \({\rm{PC}}{{\rm{l}}_{\rm{5}}}\) | P. Square pyramidal |
| B. \({\rm{S}}{{\rm{F}}_{\rm{6}}}\) | Q. Trigonal planar |
| C. \({\rm{Br}}{{\rm{F}}_{\rm{5}}}\) | R. Octahedral |
| D. \({\rm{B}}{{\rm{F}}_{\rm{3}}}\) | S. Trigonal bipyramidal |