313926
Give the correct order of initials \(\mathrm{T}\) or \(\mathrm{F}\) for following statements. Use \(\mathrm{T}\) if statement is true and \(\mathrm{F}\) if it is false
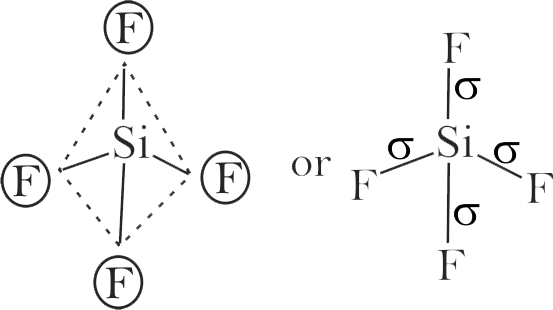
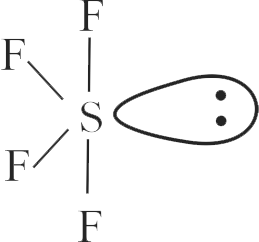
i) The order of repulsion between different pair of electrons is \({\text{1p - 1p > 1p - bp > bp - b p}}\)
ii) In general, as the number of lone pair of electrons on central atom increases, value of bond angle from normal bond angle also increases.
iii) The number of lone pairs on \(\mathrm{O}\) in \(\mathrm{H}_{2} \mathrm{O}\) is 2 while on \(\mathrm{N}\) in \(\mathrm{NH}_{3}\) is 1 .
iv) The structures of Xenon-fluorides and Xenon-oxyfluorides could not be explained on the basis of VSEPR theory.
313926
Give the correct order of initials \(\mathrm{T}\) or \(\mathrm{F}\) for following statements. Use \(\mathrm{T}\) if statement is true and \(\mathrm{F}\) if it is false
i) The order of repulsion between different pair of electrons is \({\text{1p - 1p > 1p - bp > bp - b p}}\)
ii) In general, as the number of lone pair of electrons on central atom increases, value of bond angle from normal bond angle also increases.
iii) The number of lone pairs on \(\mathrm{O}\) in \(\mathrm{H}_{2} \mathrm{O}\) is 2 while on \(\mathrm{N}\) in \(\mathrm{NH}_{3}\) is 1 .
iv) The structures of Xenon-fluorides and Xenon-oxyfluorides could not be explained on the basis of VSEPR theory.
313926
Give the correct order of initials \(\mathrm{T}\) or \(\mathrm{F}\) for following statements. Use \(\mathrm{T}\) if statement is true and \(\mathrm{F}\) if it is false
i) The order of repulsion between different pair of electrons is \({\text{1p - 1p > 1p - bp > bp - b p}}\)
ii) In general, as the number of lone pair of electrons on central atom increases, value of bond angle from normal bond angle also increases.
iii) The number of lone pairs on \(\mathrm{O}\) in \(\mathrm{H}_{2} \mathrm{O}\) is 2 while on \(\mathrm{N}\) in \(\mathrm{NH}_{3}\) is 1 .
iv) The structures of Xenon-fluorides and Xenon-oxyfluorides could not be explained on the basis of VSEPR theory.
313926
Give the correct order of initials \(\mathrm{T}\) or \(\mathrm{F}\) for following statements. Use \(\mathrm{T}\) if statement is true and \(\mathrm{F}\) if it is false
i) The order of repulsion between different pair of electrons is \({\text{1p - 1p > 1p - bp > bp - b p}}\)
ii) In general, as the number of lone pair of electrons on central atom increases, value of bond angle from normal bond angle also increases.
iii) The number of lone pairs on \(\mathrm{O}\) in \(\mathrm{H}_{2} \mathrm{O}\) is 2 while on \(\mathrm{N}\) in \(\mathrm{NH}_{3}\) is 1 .
iv) The structures of Xenon-fluorides and Xenon-oxyfluorides could not be explained on the basis of VSEPR theory.