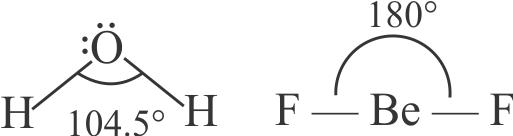313840
Given below are two statements:
Statement A :
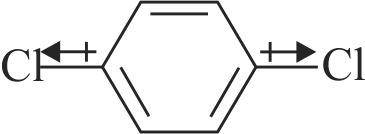
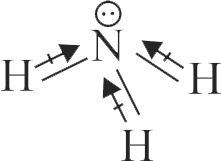
Dipole moment is a vector quantity and by convention it is depicted by a small arrow with tail on the negative centre and head pointing towards the positive centre.
Statement B :
The crossed arrow of the dipole moment symbolizes the direction of the shift of charges in the molecule.
313840
Given below are two statements:
Statement A :
Dipole moment is a vector quantity and by convention it is depicted by a small arrow with tail on the negative centre and head pointing towards the positive centre.
Statement B :
The crossed arrow of the dipole moment symbolizes the direction of the shift of charges in the molecule.
313840
Given below are two statements:
Statement A :
Dipole moment is a vector quantity and by convention it is depicted by a small arrow with tail on the negative centre and head pointing towards the positive centre.
Statement B :
The crossed arrow of the dipole moment symbolizes the direction of the shift of charges in the molecule.
313840
Given below are two statements:
Statement A :
Dipole moment is a vector quantity and by convention it is depicted by a small arrow with tail on the negative centre and head pointing towards the positive centre.
Statement B :
The crossed arrow of the dipole moment symbolizes the direction of the shift of charges in the molecule.
313840
Given below are two statements:
Statement A :
Dipole moment is a vector quantity and by convention it is depicted by a small arrow with tail on the negative centre and head pointing towards the positive centre.
Statement B :
The crossed arrow of the dipole moment symbolizes the direction of the shift of charges in the molecule.