313767
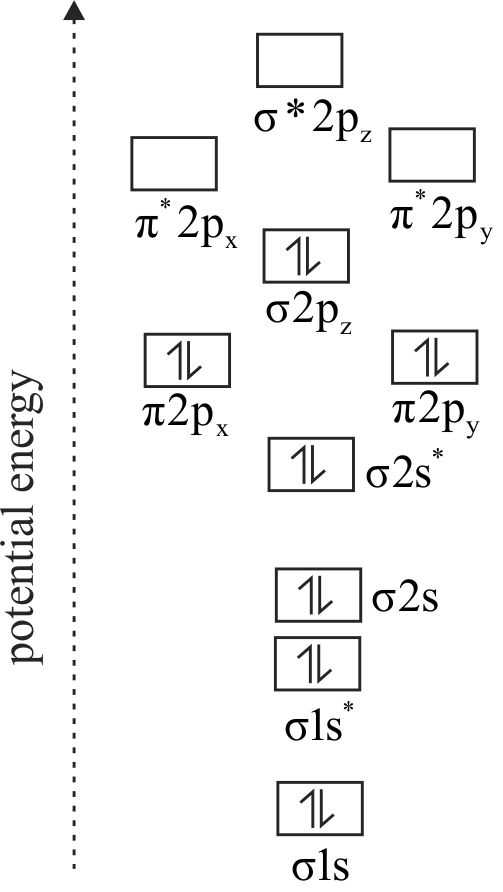
Match List-I (Molecules) with List-II (Bond order) and select the correct answer using the codes.
| Column I | Column II |
|---|---|
| A. \({\rm{L}}{{\rm{i}}_{\rm{2}}}\) | P. \({\rm{3}}\) |
| B. \({{\rm{N}}_{\rm{2}}}\) | Q. \({\rm{1.5}}\) |
| C. \({\rm{B}}{{\rm{e}}_{\rm{2}}}\) | R. \({\rm{1}}\) |
| D. \({{\rm{O}}_{\rm{2}}}\) | S. \({\rm{0}}\) |
| T. \({\rm{2}}\) |
313767
Match List-I (Molecules) with List-II (Bond order) and select the correct answer using the codes.
| Column I | Column II |
|---|---|
| A. \({\rm{L}}{{\rm{i}}_{\rm{2}}}\) | P. \({\rm{3}}\) |
| B. \({{\rm{N}}_{\rm{2}}}\) | Q. \({\rm{1.5}}\) |
| C. \({\rm{B}}{{\rm{e}}_{\rm{2}}}\) | R. \({\rm{1}}\) |
| D. \({{\rm{O}}_{\rm{2}}}\) | S. \({\rm{0}}\) |
| T. \({\rm{2}}\) |
313767
Match List-I (Molecules) with List-II (Bond order) and select the correct answer using the codes.
| Column I | Column II |
|---|---|
| A. \({\rm{L}}{{\rm{i}}_{\rm{2}}}\) | P. \({\rm{3}}\) |
| B. \({{\rm{N}}_{\rm{2}}}\) | Q. \({\rm{1.5}}\) |
| C. \({\rm{B}}{{\rm{e}}_{\rm{2}}}\) | R. \({\rm{1}}\) |
| D. \({{\rm{O}}_{\rm{2}}}\) | S. \({\rm{0}}\) |
| T. \({\rm{2}}\) |
313767
Match List-I (Molecules) with List-II (Bond order) and select the correct answer using the codes.
| Column I | Column II |
|---|---|
| A. \({\rm{L}}{{\rm{i}}_{\rm{2}}}\) | P. \({\rm{3}}\) |
| B. \({{\rm{N}}_{\rm{2}}}\) | Q. \({\rm{1.5}}\) |
| C. \({\rm{B}}{{\rm{e}}_{\rm{2}}}\) | R. \({\rm{1}}\) |
| D. \({{\rm{O}}_{\rm{2}}}\) | S. \({\rm{0}}\) |
| T. \({\rm{2}}\) |
313767
Match List-I (Molecules) with List-II (Bond order) and select the correct answer using the codes.
| Column I | Column II |
|---|---|
| A. \({\rm{L}}{{\rm{i}}_{\rm{2}}}\) | P. \({\rm{3}}\) |
| B. \({{\rm{N}}_{\rm{2}}}\) | Q. \({\rm{1.5}}\) |
| C. \({\rm{B}}{{\rm{e}}_{\rm{2}}}\) | R. \({\rm{1}}\) |
| D. \({{\rm{O}}_{\rm{2}}}\) | S. \({\rm{0}}\) |
| T. \({\rm{2}}\) |