Explanation:
Resonating structures of \({\mathrm{\mathrm{SO}_{2}}}\).
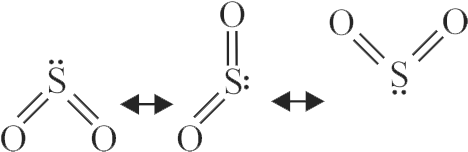
\[{\rm{Bond order = }}\frac{{{\rm{(Total}}\,{\rm{no}}{\rm{.of}}\,{\rm{bonds)}}}}{{\left( \begin{array}{l}
{\rm{Total}}\,{\rm{no}}{\rm{.of}}\\
{\rm{resonating}}\,\,{\rm{structure}}
\end{array} \right)}}\]
\(\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\, = \frac{4}{3} = 1.33\)