313456
Assertion :
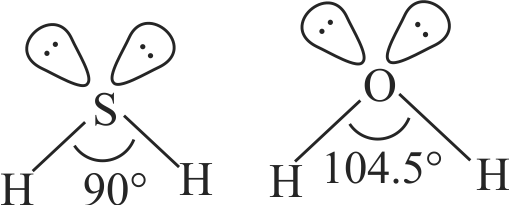
\(\mathrm{H}-\mathrm{S}-\mathrm{H}\) bond angle in \(\mathrm{H}_{2} \mathrm{~S}\) is closer to \(90^{\circ}\) but \(\mathrm{H}-\mathrm{O}-\mathrm{H}\) bond angle in \(\mathrm{H}_{2} \mathrm{O}\) is \(104.5^{\circ}\).
Reason :
Lone pair - lone pair repulsion is stronger in \(\mathrm{H}_{2} \mathrm{~S}\) than in \(\mathrm{H}_{2} \mathrm{O}\).
313456
Assertion :
\(\mathrm{H}-\mathrm{S}-\mathrm{H}\) bond angle in \(\mathrm{H}_{2} \mathrm{~S}\) is closer to \(90^{\circ}\) but \(\mathrm{H}-\mathrm{O}-\mathrm{H}\) bond angle in \(\mathrm{H}_{2} \mathrm{O}\) is \(104.5^{\circ}\).
Reason :
Lone pair - lone pair repulsion is stronger in \(\mathrm{H}_{2} \mathrm{~S}\) than in \(\mathrm{H}_{2} \mathrm{O}\).
313456
Assertion :
\(\mathrm{H}-\mathrm{S}-\mathrm{H}\) bond angle in \(\mathrm{H}_{2} \mathrm{~S}\) is closer to \(90^{\circ}\) but \(\mathrm{H}-\mathrm{O}-\mathrm{H}\) bond angle in \(\mathrm{H}_{2} \mathrm{O}\) is \(104.5^{\circ}\).
Reason :
Lone pair - lone pair repulsion is stronger in \(\mathrm{H}_{2} \mathrm{~S}\) than in \(\mathrm{H}_{2} \mathrm{O}\).
313456
Assertion :
\(\mathrm{H}-\mathrm{S}-\mathrm{H}\) bond angle in \(\mathrm{H}_{2} \mathrm{~S}\) is closer to \(90^{\circ}\) but \(\mathrm{H}-\mathrm{O}-\mathrm{H}\) bond angle in \(\mathrm{H}_{2} \mathrm{O}\) is \(104.5^{\circ}\).
Reason :
Lone pair - lone pair repulsion is stronger in \(\mathrm{H}_{2} \mathrm{~S}\) than in \(\mathrm{H}_{2} \mathrm{O}\).