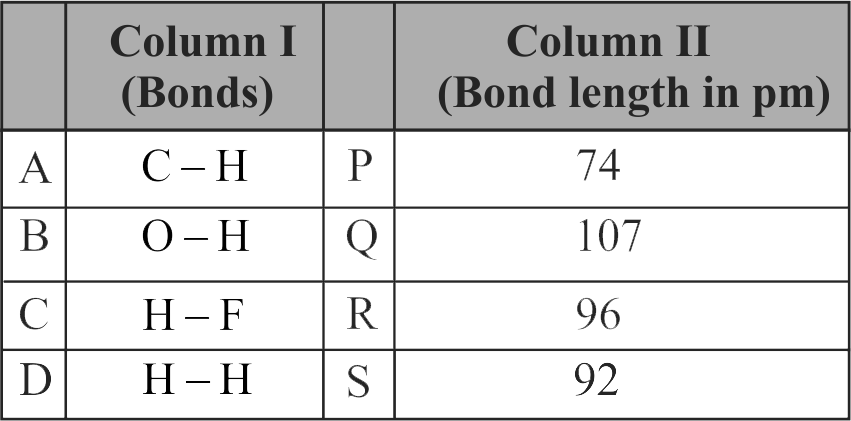
313469
In which of the following conversion, bond length increases?
(P) \(\mathrm{NO} \rightarrow \mathrm{NO}^{+}\)
(Q) \(\mathrm{N}_{2}^{+} \rightarrow \mathrm{N}_{2}^{-}\)
(R) \(\mathrm{O}_{2} \rightarrow \mathrm{O}_{2}^{+}\)
(S) \(\mathrm{H}_{2} \rightarrow \mathrm{H}_{2}^{+}\)
313471
Bond length depends upon the size of atoms, hybridisation, resonance, bond order etc. In the following relations,
I. Bond length \( \propto \) size of atoms
II. Bond length \( \propto \) hybridisation (no. of hybrid
III. Bond length \( \propto \) bond order
Choose the correct option.
313469
In which of the following conversion, bond length increases?
(P) \(\mathrm{NO} \rightarrow \mathrm{NO}^{+}\)
(Q) \(\mathrm{N}_{2}^{+} \rightarrow \mathrm{N}_{2}^{-}\)
(R) \(\mathrm{O}_{2} \rightarrow \mathrm{O}_{2}^{+}\)
(S) \(\mathrm{H}_{2} \rightarrow \mathrm{H}_{2}^{+}\)
313471
Bond length depends upon the size of atoms, hybridisation, resonance, bond order etc. In the following relations,
I. Bond length \( \propto \) size of atoms
II. Bond length \( \propto \) hybridisation (no. of hybrid
III. Bond length \( \propto \) bond order
Choose the correct option.
313469
In which of the following conversion, bond length increases?
(P) \(\mathrm{NO} \rightarrow \mathrm{NO}^{+}\)
(Q) \(\mathrm{N}_{2}^{+} \rightarrow \mathrm{N}_{2}^{-}\)
(R) \(\mathrm{O}_{2} \rightarrow \mathrm{O}_{2}^{+}\)
(S) \(\mathrm{H}_{2} \rightarrow \mathrm{H}_{2}^{+}\)
313471
Bond length depends upon the size of atoms, hybridisation, resonance, bond order etc. In the following relations,
I. Bond length \( \propto \) size of atoms
II. Bond length \( \propto \) hybridisation (no. of hybrid
III. Bond length \( \propto \) bond order
Choose the correct option.
313469
In which of the following conversion, bond length increases?
(P) \(\mathrm{NO} \rightarrow \mathrm{NO}^{+}\)
(Q) \(\mathrm{N}_{2}^{+} \rightarrow \mathrm{N}_{2}^{-}\)
(R) \(\mathrm{O}_{2} \rightarrow \mathrm{O}_{2}^{+}\)
(S) \(\mathrm{H}_{2} \rightarrow \mathrm{H}_{2}^{+}\)
313471
Bond length depends upon the size of atoms, hybridisation, resonance, bond order etc. In the following relations,
I. Bond length \( \propto \) size of atoms
II. Bond length \( \propto \) hybridisation (no. of hybrid
III. Bond length \( \propto \) bond order
Choose the correct option.
313469
In which of the following conversion, bond length increases?
(P) \(\mathrm{NO} \rightarrow \mathrm{NO}^{+}\)
(Q) \(\mathrm{N}_{2}^{+} \rightarrow \mathrm{N}_{2}^{-}\)
(R) \(\mathrm{O}_{2} \rightarrow \mathrm{O}_{2}^{+}\)
(S) \(\mathrm{H}_{2} \rightarrow \mathrm{H}_{2}^{+}\)
313471
Bond length depends upon the size of atoms, hybridisation, resonance, bond order etc. In the following relations,
I. Bond length \( \propto \) size of atoms
II. Bond length \( \propto \) hybridisation (no. of hybrid
III. Bond length \( \propto \) bond order
Choose the correct option.