CHXI02:STRUCTURE OF ATOM
307172
Match Column I with Column II and choose the correct combination from the options given.
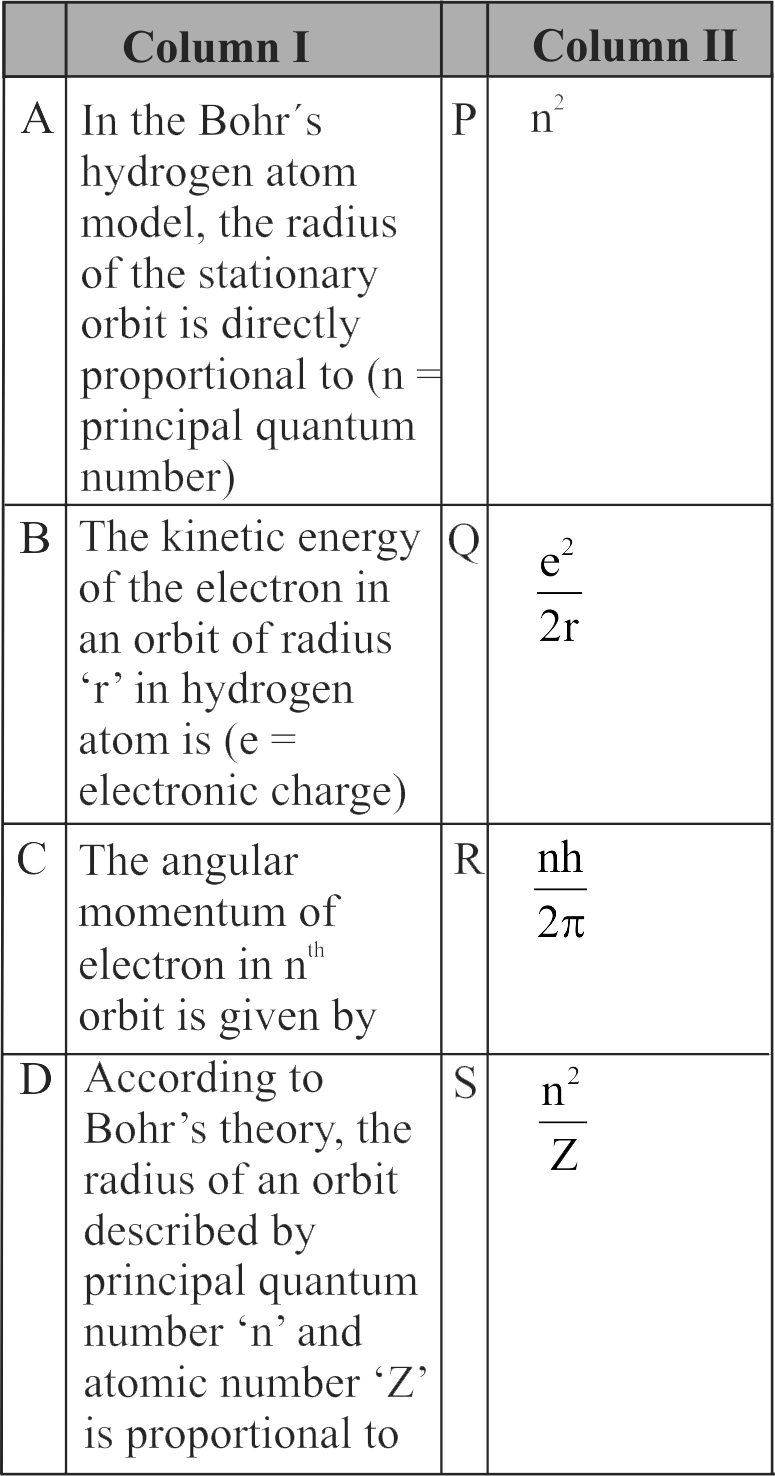
1 \((1){\text{A}} - {\text{P}},{\text{B}} - {\text{R}},{\text{C}} - {\text{S}},{\text{D}} - {\text{Q}},{\text{R}}\)
2 \((2)\,{\rm{A}} - {\rm{P}},{\rm{ B}} - {\rm{Q}},{\rm{ C}} - {\rm{R}},{\rm{ D}} - {\rm{S}}\)
3 \((3)\,{\rm{A}} - {\rm{Q}},{\rm{ B}} - {\rm{P}},{\rm{ C}} - {\rm{S}},{\rm{ D}} - {\rm{R}}\)
4 \((4)\,{\mkern 1mu} {\text{A}} - {\text{P}},{\text{B}} - {\text{R}},{\text{C}} - {\text{S}},{\text{D}} - {\text{R}}\)
Explanation:
\(r\,\alpha \,{n^2}\) (for hydrogen atom)
\(\mathrm{Ek}=\dfrac{\mathrm{e}^{2}}{2 \mathrm{r}}\)
\(\mathrm{mvr}=\mathrm{n} \dfrac{\mathrm{h}}{2 \pi}\)
radius, \(\mathrm{r}=0.53 \times \dfrac{\mathrm{n}^{2}}{\mathrm{z}}\)