356581
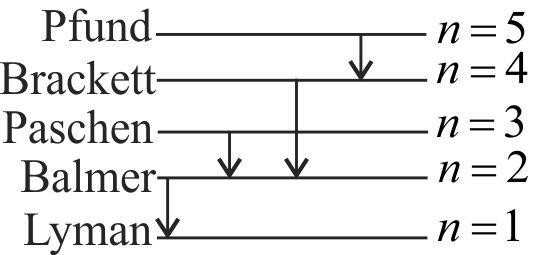
The Balmer series for the \(H\)-atom can be observed,
I.
If we measure the frequencies of light emitted when an excited atom falls to ground state
II.
If we measure the frequencies of light emitted due to transition between excited states and first excited state
III.
In any transition of a \(H\)-atom
IV.
Frequencies of higher values will be closely packed
Choose the correct option from the codes given below
356581
The Balmer series for the \(H\)-atom can be observed,
I.
If we measure the frequencies of light emitted when an excited atom falls to ground state
II.
If we measure the frequencies of light emitted due to transition between excited states and first excited state
III.
In any transition of a \(H\)-atom
IV.
Frequencies of higher values will be closely packed
Choose the correct option from the codes given below
356581
The Balmer series for the \(H\)-atom can be observed,
I.
If we measure the frequencies of light emitted when an excited atom falls to ground state
II.
If we measure the frequencies of light emitted due to transition between excited states and first excited state
III.
In any transition of a \(H\)-atom
IV.
Frequencies of higher values will be closely packed
Choose the correct option from the codes given below
356581
The Balmer series for the \(H\)-atom can be observed,
I.
If we measure the frequencies of light emitted when an excited atom falls to ground state
II.
If we measure the frequencies of light emitted due to transition between excited states and first excited state
III.
In any transition of a \(H\)-atom
IV.
Frequencies of higher values will be closely packed
Choose the correct option from the codes given below
356581
The Balmer series for the \(H\)-atom can be observed,
I.
If we measure the frequencies of light emitted when an excited atom falls to ground state
II.
If we measure the frequencies of light emitted due to transition between excited states and first excited state
III.
In any transition of a \(H\)-atom
IV.
Frequencies of higher values will be closely packed
Choose the correct option from the codes given below