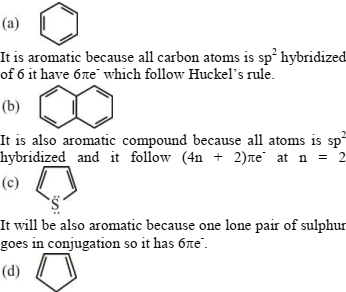
232334 $\mathrm{C}_{6} \mathrm{H}_{6}$ consist of one ring, while naphthalene consists of two rings both of them are aromatic and obey the $(4 n+2 \pi)$ rule Thus the number of $\pi$-electrons inside rings of $\mathrm{C}_{6} \mathrm{H}_{6}$ and naphthalene are respectively:
232334 $\mathrm{C}_{6} \mathrm{H}_{6}$ consist of one ring, while naphthalene consists of two rings both of them are aromatic and obey the $(4 n+2 \pi)$ rule Thus the number of $\pi$-electrons inside rings of $\mathrm{C}_{6} \mathrm{H}_{6}$ and naphthalene are respectively:
232334 $\mathrm{C}_{6} \mathrm{H}_{6}$ consist of one ring, while naphthalene consists of two rings both of them are aromatic and obey the $(4 n+2 \pi)$ rule Thus the number of $\pi$-electrons inside rings of $\mathrm{C}_{6} \mathrm{H}_{6}$ and naphthalene are respectively:
232334 $\mathrm{C}_{6} \mathrm{H}_{6}$ consist of one ring, while naphthalene consists of two rings both of them are aromatic and obey the $(4 n+2 \pi)$ rule Thus the number of $\pi$-electrons inside rings of $\mathrm{C}_{6} \mathrm{H}_{6}$ and naphthalene are respectively: