231752
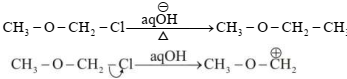
\(\text{C}{{\text{H}}_{3}}-\text{O}-\text{C}{{\text{H}}_{2}}-\text{Cl}\xrightarrow[\Delta ]{\text{ aq}\text{.}\,\overset{-}{\mathop{\text{O}}}\,\text{H }}\text{ C}{{\text{H}}_{3}}-\text{O}-\text{C}{{\text{H}}_{2}}-\text{OH}\)
Which information below regarding this reaction is applicable?
231752
\(\text{C}{{\text{H}}_{3}}-\text{O}-\text{C}{{\text{H}}_{2}}-\text{Cl}\xrightarrow[\Delta ]{\text{ aq}\text{.}\,\overset{-}{\mathop{\text{O}}}\,\text{H }}\text{ C}{{\text{H}}_{3}}-\text{O}-\text{C}{{\text{H}}_{2}}-\text{OH}\)
Which information below regarding this reaction is applicable?
231752
\(\text{C}{{\text{H}}_{3}}-\text{O}-\text{C}{{\text{H}}_{2}}-\text{Cl}\xrightarrow[\Delta ]{\text{ aq}\text{.}\,\overset{-}{\mathop{\text{O}}}\,\text{H }}\text{ C}{{\text{H}}_{3}}-\text{O}-\text{C}{{\text{H}}_{2}}-\text{OH}\)
Which information below regarding this reaction is applicable?
231752
\(\text{C}{{\text{H}}_{3}}-\text{O}-\text{C}{{\text{H}}_{2}}-\text{Cl}\xrightarrow[\Delta ]{\text{ aq}\text{.}\,\overset{-}{\mathop{\text{O}}}\,\text{H }}\text{ C}{{\text{H}}_{3}}-\text{O}-\text{C}{{\text{H}}_{2}}-\text{OH}\)
Which information below regarding this reaction is applicable?