231678
Arrange the following in increasing order of stability on the basis of reasonance.
(i) \(\mathrm{CH}_2=\mathrm{CH}-\mathrm{CH}=\mathrm{CH}-\stackrel{+}{\mathrm{H}}_2\)
(ii) \(\mathrm{CH}_2=\mathrm{CH}-\stackrel{+}{\mathrm{CH}}-\mathrm{CH}=\mathrm{CH}_2\)
(iii) \(\mathrm{CH}_2=\mathrm{CH}-\stackrel{+}{\mathrm{CH}}-\stackrel{-}{\mathrm{C}}-\stackrel{+}{\mathrm{C}}_2\)
(iv) \(\stackrel{+}{\mathrm{CH}}_2-\mathrm{CH}=\mathrm{CH}-\stackrel{-}{\mathrm{CH}}-\stackrel{+}{\mathrm{CH}}_2\)
231678
Arrange the following in increasing order of stability on the basis of reasonance.
(i) \(\mathrm{CH}_2=\mathrm{CH}-\mathrm{CH}=\mathrm{CH}-\stackrel{+}{\mathrm{H}}_2\)
(ii) \(\mathrm{CH}_2=\mathrm{CH}-\stackrel{+}{\mathrm{CH}}-\mathrm{CH}=\mathrm{CH}_2\)
(iii) \(\mathrm{CH}_2=\mathrm{CH}-\stackrel{+}{\mathrm{CH}}-\stackrel{-}{\mathrm{C}}-\stackrel{+}{\mathrm{C}}_2\)
(iv) \(\stackrel{+}{\mathrm{CH}}_2-\mathrm{CH}=\mathrm{CH}-\stackrel{-}{\mathrm{CH}}-\stackrel{+}{\mathrm{CH}}_2\)
231678
Arrange the following in increasing order of stability on the basis of reasonance.
(i) \(\mathrm{CH}_2=\mathrm{CH}-\mathrm{CH}=\mathrm{CH}-\stackrel{+}{\mathrm{H}}_2\)
(ii) \(\mathrm{CH}_2=\mathrm{CH}-\stackrel{+}{\mathrm{CH}}-\mathrm{CH}=\mathrm{CH}_2\)
(iii) \(\mathrm{CH}_2=\mathrm{CH}-\stackrel{+}{\mathrm{CH}}-\stackrel{-}{\mathrm{C}}-\stackrel{+}{\mathrm{C}}_2\)
(iv) \(\stackrel{+}{\mathrm{CH}}_2-\mathrm{CH}=\mathrm{CH}-\stackrel{-}{\mathrm{CH}}-\stackrel{+}{\mathrm{CH}}_2\)
231678
Arrange the following in increasing order of stability on the basis of reasonance.
(i) \(\mathrm{CH}_2=\mathrm{CH}-\mathrm{CH}=\mathrm{CH}-\stackrel{+}{\mathrm{H}}_2\)
(ii) \(\mathrm{CH}_2=\mathrm{CH}-\stackrel{+}{\mathrm{CH}}-\mathrm{CH}=\mathrm{CH}_2\)
(iii) \(\mathrm{CH}_2=\mathrm{CH}-\stackrel{+}{\mathrm{CH}}-\stackrel{-}{\mathrm{C}}-\stackrel{+}{\mathrm{C}}_2\)
(iv) \(\stackrel{+}{\mathrm{CH}}_2-\mathrm{CH}=\mathrm{CH}-\stackrel{-}{\mathrm{CH}}-\stackrel{+}{\mathrm{CH}}_2\)
231678
Arrange the following in increasing order of stability on the basis of reasonance.
(i) \(\mathrm{CH}_2=\mathrm{CH}-\mathrm{CH}=\mathrm{CH}-\stackrel{+}{\mathrm{H}}_2\)
(ii) \(\mathrm{CH}_2=\mathrm{CH}-\stackrel{+}{\mathrm{CH}}-\mathrm{CH}=\mathrm{CH}_2\)
(iii) \(\mathrm{CH}_2=\mathrm{CH}-\stackrel{+}{\mathrm{CH}}-\stackrel{-}{\mathrm{C}}-\stackrel{+}{\mathrm{C}}_2\)
(iv) \(\stackrel{+}{\mathrm{CH}}_2-\mathrm{CH}=\mathrm{CH}-\stackrel{-}{\mathrm{CH}}-\stackrel{+}{\mathrm{CH}}_2\)




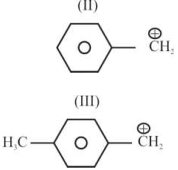

 So, $\underline{\mathrm{ii}}>\mathrm{i}>\mathrm{iii}>\mathrm{iv}$ is the increasing order of stability.
So, $\underline{\mathrm{ii}}>\mathrm{i}>\mathrm{iii}>\mathrm{iv}$ is the increasing order of stability.