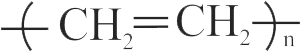324968
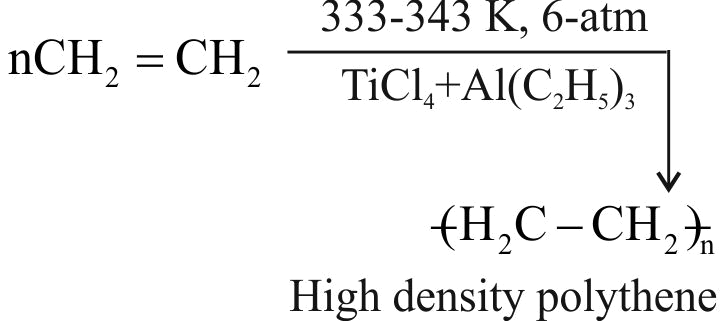
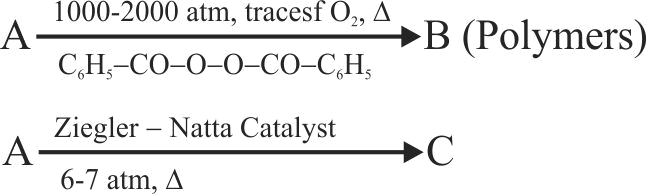
The formation of polyethylene from \({\mathrm{\mathrm{CaC}_{2}}}\) is as:
\({{\rm{Ca}}{{\rm{C}}_2} + 2{{\rm{H}}_2}{\rm{O}} \to {\rm{Ca}}{{({\rm{OH}})}_2} + {{\rm{C}}_2}{{\rm{H}}_2}}\)
\({{{\rm{C}}_2}{{\rm{H}}_2} + {{\rm{H}}_2} \to {{\rm{C}}_2}{{\rm{H}}_4}}\)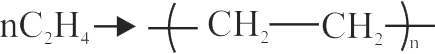
The amount of polyethylene obtained from 12.8 kg of \({\mathrm{\mathrm{CaC}_{2}}}\) is ____ .
324968
The formation of polyethylene from \({\mathrm{\mathrm{CaC}_{2}}}\) is as:
\({{\rm{Ca}}{{\rm{C}}_2} + 2{{\rm{H}}_2}{\rm{O}} \to {\rm{Ca}}{{({\rm{OH}})}_2} + {{\rm{C}}_2}{{\rm{H}}_2}}\)
\({{{\rm{C}}_2}{{\rm{H}}_2} + {{\rm{H}}_2} \to {{\rm{C}}_2}{{\rm{H}}_4}}\)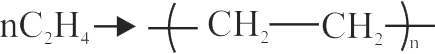
The amount of polyethylene obtained from 12.8 kg of \({\mathrm{\mathrm{CaC}_{2}}}\) is ____ .
324968
The formation of polyethylene from \({\mathrm{\mathrm{CaC}_{2}}}\) is as:
\({{\rm{Ca}}{{\rm{C}}_2} + 2{{\rm{H}}_2}{\rm{O}} \to {\rm{Ca}}{{({\rm{OH}})}_2} + {{\rm{C}}_2}{{\rm{H}}_2}}\)
\({{{\rm{C}}_2}{{\rm{H}}_2} + {{\rm{H}}_2} \to {{\rm{C}}_2}{{\rm{H}}_4}}\)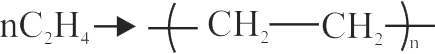
The amount of polyethylene obtained from 12.8 kg of \({\mathrm{\mathrm{CaC}_{2}}}\) is ____ .
324968
The formation of polyethylene from \({\mathrm{\mathrm{CaC}_{2}}}\) is as:
\({{\rm{Ca}}{{\rm{C}}_2} + 2{{\rm{H}}_2}{\rm{O}} \to {\rm{Ca}}{{({\rm{OH}})}_2} + {{\rm{C}}_2}{{\rm{H}}_2}}\)
\({{{\rm{C}}_2}{{\rm{H}}_2} + {{\rm{H}}_2} \to {{\rm{C}}_2}{{\rm{H}}_4}}\)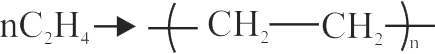
The amount of polyethylene obtained from 12.8 kg of \({\mathrm{\mathrm{CaC}_{2}}}\) is ____ .