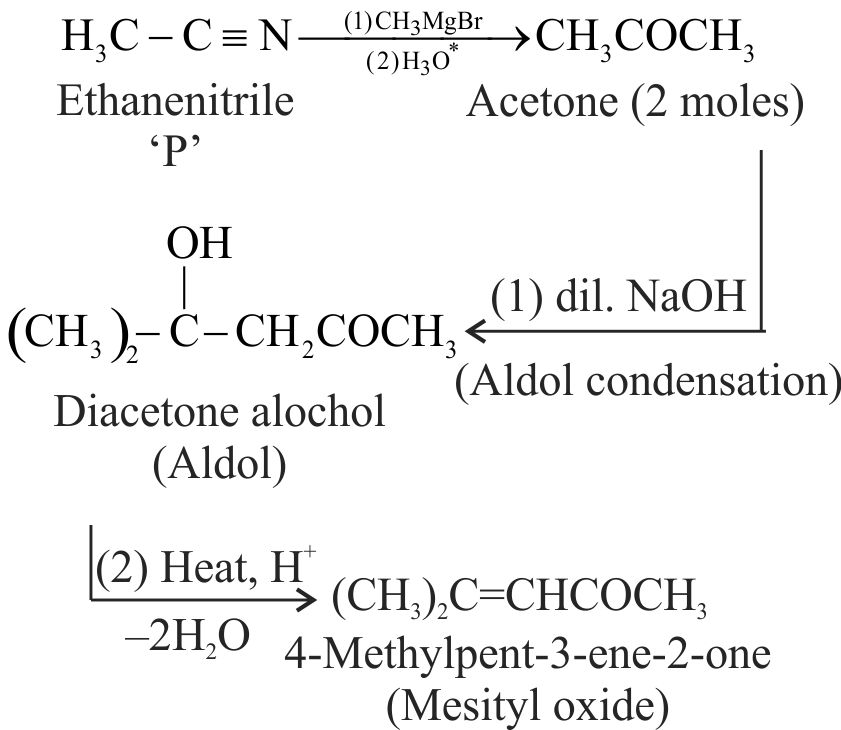
324301 \({\text{P}}\xrightarrow[{{\text{2}}{\text{.}}{{\text{H}}_{\text{2}}}{{\text{O}}^{\text{*}}}}]{{{\text{1}}{\text{.C}}{{\text{H}}_{\text{3}}}{\text{MgBr}}}}{\text{R}}\xrightarrow[{{\text{2}}.\Delta }]{{{\text{1}}{\text{.dil}}{\text{. NaOH}}}}\) 4-methylpent-3-en-one \(\mathrm{P}\) is
324301 \({\text{P}}\xrightarrow[{{\text{2}}{\text{.}}{{\text{H}}_{\text{2}}}{{\text{O}}^{\text{*}}}}]{{{\text{1}}{\text{.C}}{{\text{H}}_{\text{3}}}{\text{MgBr}}}}{\text{R}}\xrightarrow[{{\text{2}}.\Delta }]{{{\text{1}}{\text{.dil}}{\text{. NaOH}}}}\) 4-methylpent-3-en-one \(\mathrm{P}\) is
324301 \({\text{P}}\xrightarrow[{{\text{2}}{\text{.}}{{\text{H}}_{\text{2}}}{{\text{O}}^{\text{*}}}}]{{{\text{1}}{\text{.C}}{{\text{H}}_{\text{3}}}{\text{MgBr}}}}{\text{R}}\xrightarrow[{{\text{2}}.\Delta }]{{{\text{1}}{\text{.dil}}{\text{. NaOH}}}}\) 4-methylpent-3-en-one \(\mathrm{P}\) is
324301 \({\text{P}}\xrightarrow[{{\text{2}}{\text{.}}{{\text{H}}_{\text{2}}}{{\text{O}}^{\text{*}}}}]{{{\text{1}}{\text{.C}}{{\text{H}}_{\text{3}}}{\text{MgBr}}}}{\text{R}}\xrightarrow[{{\text{2}}.\Delta }]{{{\text{1}}{\text{.dil}}{\text{. NaOH}}}}\) 4-methylpent-3-en-one \(\mathrm{P}\) is
324301 \({\text{P}}\xrightarrow[{{\text{2}}{\text{.}}{{\text{H}}_{\text{2}}}{{\text{O}}^{\text{*}}}}]{{{\text{1}}{\text{.C}}{{\text{H}}_{\text{3}}}{\text{MgBr}}}}{\text{R}}\xrightarrow[{{\text{2}}.\Delta }]{{{\text{1}}{\text{.dil}}{\text{. NaOH}}}}\) 4-methylpent-3-en-one \(\mathrm{P}\) is