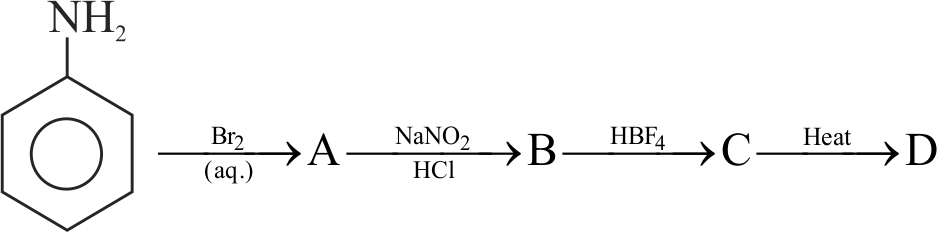
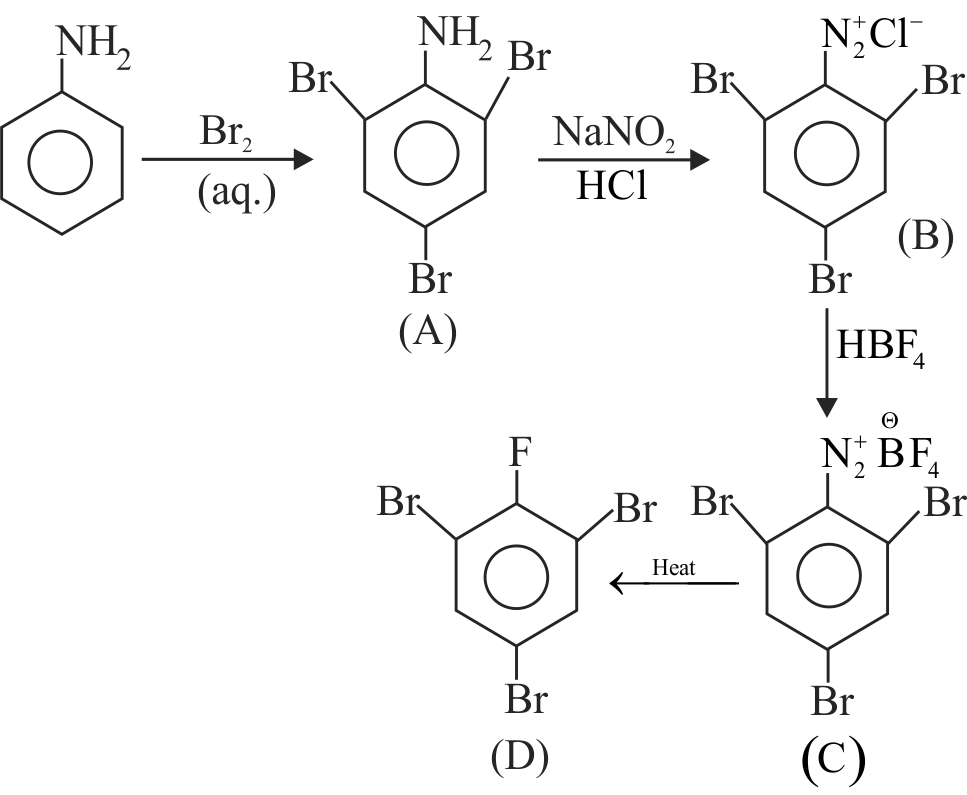
324266
\({\text{Ca}}{{\text{C}}_{\text{2}}}\xrightarrow[{{\text{ - Ca(OH}}{{\text{)}}_{\text{2}}}}]{{{\text{Hydrolysis}}}}{\text{A}}\xrightarrow[{{\text{Fe tube}}}]{{{\text{Red}}\,\,{\text{hot}}}}{\text{B}}\)
\(\xrightarrow[{{\text{50 - 6}}{{\text{0}}^{\text{o}}}\,{\text{C}}}]{{{\text{HN}}{{\text{O}}_{\text{3}}}{\text{ + }}{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}}}{\text{C}}\)
\(\xrightarrow{{{\text{Fe + HCl}}}}{\text{D}}\xrightarrow{{{\text{NaN}}{{\text{O}}_{\text{2}}}{\text{ + HCl,}}{{\text{O}}^{\text{o}}}{\text{C}}}}{\text{E}}\)
Then \(\mathrm{E}\) is
324266
\({\text{Ca}}{{\text{C}}_{\text{2}}}\xrightarrow[{{\text{ - Ca(OH}}{{\text{)}}_{\text{2}}}}]{{{\text{Hydrolysis}}}}{\text{A}}\xrightarrow[{{\text{Fe tube}}}]{{{\text{Red}}\,\,{\text{hot}}}}{\text{B}}\)
\(\xrightarrow[{{\text{50 - 6}}{{\text{0}}^{\text{o}}}\,{\text{C}}}]{{{\text{HN}}{{\text{O}}_{\text{3}}}{\text{ + }}{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}}}{\text{C}}\)
\(\xrightarrow{{{\text{Fe + HCl}}}}{\text{D}}\xrightarrow{{{\text{NaN}}{{\text{O}}_{\text{2}}}{\text{ + HCl,}}{{\text{O}}^{\text{o}}}{\text{C}}}}{\text{E}}\)
Then \(\mathrm{E}\) is
324266
\({\text{Ca}}{{\text{C}}_{\text{2}}}\xrightarrow[{{\text{ - Ca(OH}}{{\text{)}}_{\text{2}}}}]{{{\text{Hydrolysis}}}}{\text{A}}\xrightarrow[{{\text{Fe tube}}}]{{{\text{Red}}\,\,{\text{hot}}}}{\text{B}}\)
\(\xrightarrow[{{\text{50 - 6}}{{\text{0}}^{\text{o}}}\,{\text{C}}}]{{{\text{HN}}{{\text{O}}_{\text{3}}}{\text{ + }}{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}}}{\text{C}}\)
\(\xrightarrow{{{\text{Fe + HCl}}}}{\text{D}}\xrightarrow{{{\text{NaN}}{{\text{O}}_{\text{2}}}{\text{ + HCl,}}{{\text{O}}^{\text{o}}}{\text{C}}}}{\text{E}}\)
Then \(\mathrm{E}\) is
324266
\({\text{Ca}}{{\text{C}}_{\text{2}}}\xrightarrow[{{\text{ - Ca(OH}}{{\text{)}}_{\text{2}}}}]{{{\text{Hydrolysis}}}}{\text{A}}\xrightarrow[{{\text{Fe tube}}}]{{{\text{Red}}\,\,{\text{hot}}}}{\text{B}}\)
\(\xrightarrow[{{\text{50 - 6}}{{\text{0}}^{\text{o}}}\,{\text{C}}}]{{{\text{HN}}{{\text{O}}_{\text{3}}}{\text{ + }}{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}}}{\text{C}}\)
\(\xrightarrow{{{\text{Fe + HCl}}}}{\text{D}}\xrightarrow{{{\text{NaN}}{{\text{O}}_{\text{2}}}{\text{ + HCl,}}{{\text{O}}^{\text{o}}}{\text{C}}}}{\text{E}}\)
Then \(\mathrm{E}\) is