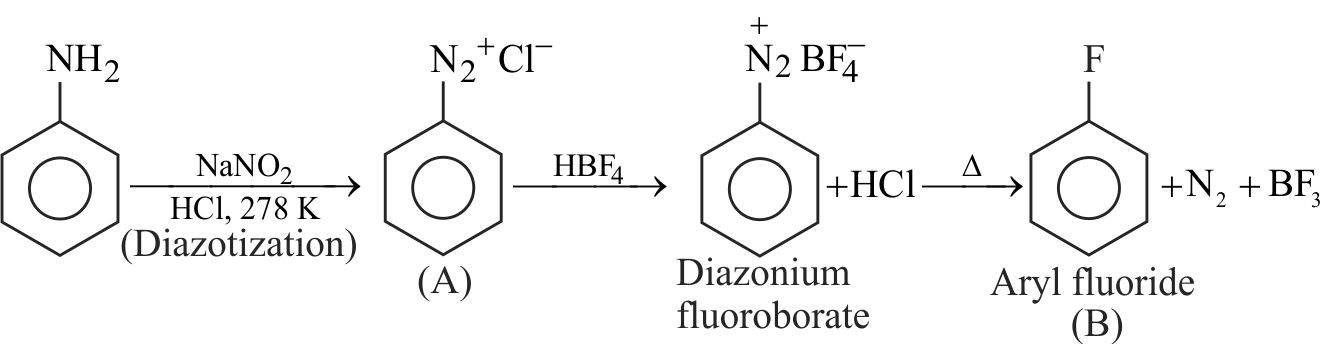
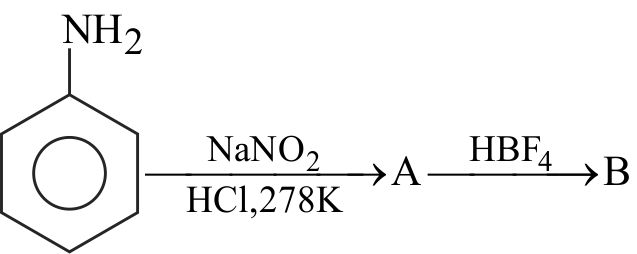324243
I. Diazonium salts are very good intermediates for the introduction of \(\mathrm{F}, \mathrm{Cl}, \mathrm{Br}, \mathrm{I}, \mathrm{CN}, \mathrm{OH}, \mathrm{NO}_{2}\) groups into the aromatic ring.
II. Aryl fluorides and iodides can be prepared by direct halogenation.
III. Cyano group can be introduced by nucleophilic substitution of chlorine in chlorobenzene easily.
IV. The replacement of the diazo group by other groups is helpful in preparing those substituted aromatic compounds which cannot be prepared by direct substitution in benzene or substituted benzene.
Select the incorrect statements.
324245
Assertion :
Aniline on reaction with \(\mathrm{NaNO}_{2} / \mathrm{HCl}\) and \(0^{\circ} \mathrm{C}\) followed by coupling with \(\beta\)-naphthol gives a dark blue coloured precipitate.
Reason :
The colour of the compound formed in the reaction of aniline with \(\mathrm{NaNO}_{2} / \mathrm{HCl}\) at \(0^{\circ} \mathrm{C}\) followed by coupling with \(\beta\)-naphthol is due to the extended conjugation.
324247
In the reaction,
\({{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}_{{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\text{0 - }}{{\text{5}}^o}{\text{C}}}^{\xrightarrow{{{\text{NaN}}{{\text{O}}_{\text{2}}}{\text{ + HCl}}}}}\left( {\text{A}} \right)_{{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\text{KCN}}}^{\xrightarrow{{{\text{C}}{{\text{u}}_{\text{2}}}{{\left( {{\text{CN}}} \right)}_{\text{2}}}}}}\left( {\text{B}} \right)\xrightarrow{{{{\text{H}}^{{\text{ + }}}}{\text{/}}{{\text{H}}_{\text{2}}}{\text{O}}}}\left( {\text{C}} \right)\).
The product (C) is
324243
I. Diazonium salts are very good intermediates for the introduction of \(\mathrm{F}, \mathrm{Cl}, \mathrm{Br}, \mathrm{I}, \mathrm{CN}, \mathrm{OH}, \mathrm{NO}_{2}\) groups into the aromatic ring.
II. Aryl fluorides and iodides can be prepared by direct halogenation.
III. Cyano group can be introduced by nucleophilic substitution of chlorine in chlorobenzene easily.
IV. The replacement of the diazo group by other groups is helpful in preparing those substituted aromatic compounds which cannot be prepared by direct substitution in benzene or substituted benzene.
Select the incorrect statements.
324245
Assertion :
Aniline on reaction with \(\mathrm{NaNO}_{2} / \mathrm{HCl}\) and \(0^{\circ} \mathrm{C}\) followed by coupling with \(\beta\)-naphthol gives a dark blue coloured precipitate.
Reason :
The colour of the compound formed in the reaction of aniline with \(\mathrm{NaNO}_{2} / \mathrm{HCl}\) at \(0^{\circ} \mathrm{C}\) followed by coupling with \(\beta\)-naphthol is due to the extended conjugation.
324247
In the reaction,
\({{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}_{{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\text{0 - }}{{\text{5}}^o}{\text{C}}}^{\xrightarrow{{{\text{NaN}}{{\text{O}}_{\text{2}}}{\text{ + HCl}}}}}\left( {\text{A}} \right)_{{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\text{KCN}}}^{\xrightarrow{{{\text{C}}{{\text{u}}_{\text{2}}}{{\left( {{\text{CN}}} \right)}_{\text{2}}}}}}\left( {\text{B}} \right)\xrightarrow{{{{\text{H}}^{{\text{ + }}}}{\text{/}}{{\text{H}}_{\text{2}}}{\text{O}}}}\left( {\text{C}} \right)\).
The product (C) is
324243
I. Diazonium salts are very good intermediates for the introduction of \(\mathrm{F}, \mathrm{Cl}, \mathrm{Br}, \mathrm{I}, \mathrm{CN}, \mathrm{OH}, \mathrm{NO}_{2}\) groups into the aromatic ring.
II. Aryl fluorides and iodides can be prepared by direct halogenation.
III. Cyano group can be introduced by nucleophilic substitution of chlorine in chlorobenzene easily.
IV. The replacement of the diazo group by other groups is helpful in preparing those substituted aromatic compounds which cannot be prepared by direct substitution in benzene or substituted benzene.
Select the incorrect statements.
324245
Assertion :
Aniline on reaction with \(\mathrm{NaNO}_{2} / \mathrm{HCl}\) and \(0^{\circ} \mathrm{C}\) followed by coupling with \(\beta\)-naphthol gives a dark blue coloured precipitate.
Reason :
The colour of the compound formed in the reaction of aniline with \(\mathrm{NaNO}_{2} / \mathrm{HCl}\) at \(0^{\circ} \mathrm{C}\) followed by coupling with \(\beta\)-naphthol is due to the extended conjugation.
324247
In the reaction,
\({{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}_{{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\text{0 - }}{{\text{5}}^o}{\text{C}}}^{\xrightarrow{{{\text{NaN}}{{\text{O}}_{\text{2}}}{\text{ + HCl}}}}}\left( {\text{A}} \right)_{{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\text{KCN}}}^{\xrightarrow{{{\text{C}}{{\text{u}}_{\text{2}}}{{\left( {{\text{CN}}} \right)}_{\text{2}}}}}}\left( {\text{B}} \right)\xrightarrow{{{{\text{H}}^{{\text{ + }}}}{\text{/}}{{\text{H}}_{\text{2}}}{\text{O}}}}\left( {\text{C}} \right)\).
The product (C) is
324243
I. Diazonium salts are very good intermediates for the introduction of \(\mathrm{F}, \mathrm{Cl}, \mathrm{Br}, \mathrm{I}, \mathrm{CN}, \mathrm{OH}, \mathrm{NO}_{2}\) groups into the aromatic ring.
II. Aryl fluorides and iodides can be prepared by direct halogenation.
III. Cyano group can be introduced by nucleophilic substitution of chlorine in chlorobenzene easily.
IV. The replacement of the diazo group by other groups is helpful in preparing those substituted aromatic compounds which cannot be prepared by direct substitution in benzene or substituted benzene.
Select the incorrect statements.
324245
Assertion :
Aniline on reaction with \(\mathrm{NaNO}_{2} / \mathrm{HCl}\) and \(0^{\circ} \mathrm{C}\) followed by coupling with \(\beta\)-naphthol gives a dark blue coloured precipitate.
Reason :
The colour of the compound formed in the reaction of aniline with \(\mathrm{NaNO}_{2} / \mathrm{HCl}\) at \(0^{\circ} \mathrm{C}\) followed by coupling with \(\beta\)-naphthol is due to the extended conjugation.
324247
In the reaction,
\({{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}_{{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\text{0 - }}{{\text{5}}^o}{\text{C}}}^{\xrightarrow{{{\text{NaN}}{{\text{O}}_{\text{2}}}{\text{ + HCl}}}}}\left( {\text{A}} \right)_{{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\text{KCN}}}^{\xrightarrow{{{\text{C}}{{\text{u}}_{\text{2}}}{{\left( {{\text{CN}}} \right)}_{\text{2}}}}}}\left( {\text{B}} \right)\xrightarrow{{{{\text{H}}^{{\text{ + }}}}{\text{/}}{{\text{H}}_{\text{2}}}{\text{O}}}}\left( {\text{C}} \right)\).
The product (C) is
324243
I. Diazonium salts are very good intermediates for the introduction of \(\mathrm{F}, \mathrm{Cl}, \mathrm{Br}, \mathrm{I}, \mathrm{CN}, \mathrm{OH}, \mathrm{NO}_{2}\) groups into the aromatic ring.
II. Aryl fluorides and iodides can be prepared by direct halogenation.
III. Cyano group can be introduced by nucleophilic substitution of chlorine in chlorobenzene easily.
IV. The replacement of the diazo group by other groups is helpful in preparing those substituted aromatic compounds which cannot be prepared by direct substitution in benzene or substituted benzene.
Select the incorrect statements.
324245
Assertion :
Aniline on reaction with \(\mathrm{NaNO}_{2} / \mathrm{HCl}\) and \(0^{\circ} \mathrm{C}\) followed by coupling with \(\beta\)-naphthol gives a dark blue coloured precipitate.
Reason :
The colour of the compound formed in the reaction of aniline with \(\mathrm{NaNO}_{2} / \mathrm{HCl}\) at \(0^{\circ} \mathrm{C}\) followed by coupling with \(\beta\)-naphthol is due to the extended conjugation.
324247
In the reaction,
\({{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}_{{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\text{0 - }}{{\text{5}}^o}{\text{C}}}^{\xrightarrow{{{\text{NaN}}{{\text{O}}_{\text{2}}}{\text{ + HCl}}}}}\left( {\text{A}} \right)_{{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\text{KCN}}}^{\xrightarrow{{{\text{C}}{{\text{u}}_{\text{2}}}{{\left( {{\text{CN}}} \right)}_{\text{2}}}}}}\left( {\text{B}} \right)\xrightarrow{{{{\text{H}}^{{\text{ + }}}}{\text{/}}{{\text{H}}_{\text{2}}}{\text{O}}}}\left( {\text{C}} \right)\).
The product (C) is