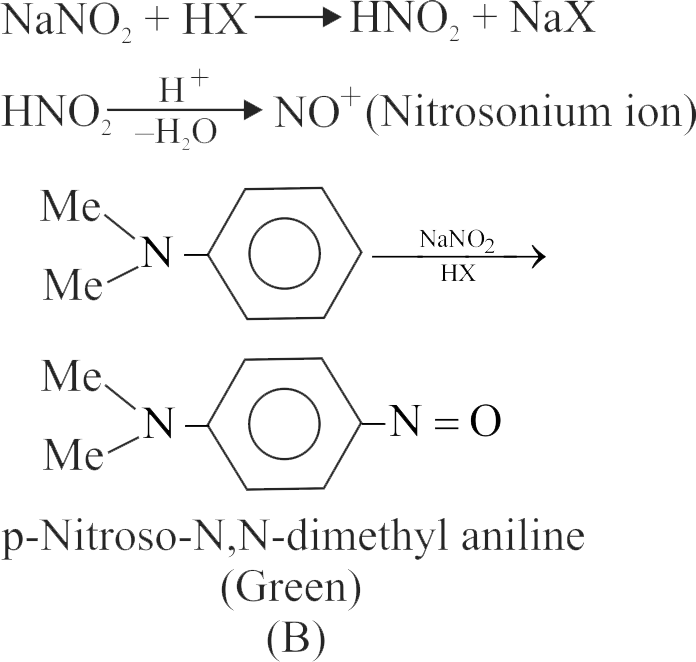
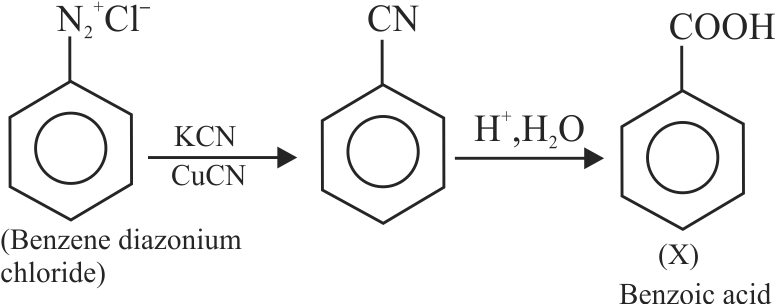
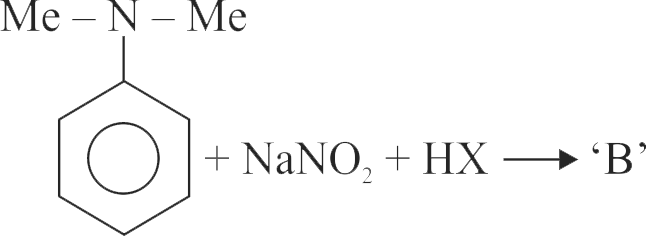324150
The correct match of Column I (Amines) and Column II (Property) is
Column I
Column II
A
\({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{N}}{{\text{H}}_{\text{2}}}\)
P
Undergoes Lieberman nitrosoamine
B
\({{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}\)
Q
Undergoes Hoffmann bromamide reaction
C
\({{\text{(C}}{{\text{H}}_{\text{3}}}{\text{)}}_{\text{2}}}{\text{NH}}\)
R
Gives azo dye test
D
\({\text{C}}{{\text{H}}_{\text{3}}}{\text{CON}}{{\text{H}}_{\text{2}}}\)
S
With alcoholic KOH and \({\text{CHC}}{{\text{l}}_{\text{3}}}\) producesbad smell
324151
A compound of molecular formulae \({{\rm{C}}_{\rm{3}}}{{\rm{H}}_{\rm{6}}}{\rm{N}}\) shows following characteristics
(i) Get dissolved in acidic medium.
(ii) Does not react with benzoylchloride
(iii) Does not give carbylamine test
(iv) Does not evolute nitrogen gas on reacting with \(\mathrm{HNO}_{2}\)
The structure of the compound is
324150
The correct match of Column I (Amines) and Column II (Property) is
Column I
Column II
A
\({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{N}}{{\text{H}}_{\text{2}}}\)
P
Undergoes Lieberman nitrosoamine
B
\({{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}\)
Q
Undergoes Hoffmann bromamide reaction
C
\({{\text{(C}}{{\text{H}}_{\text{3}}}{\text{)}}_{\text{2}}}{\text{NH}}\)
R
Gives azo dye test
D
\({\text{C}}{{\text{H}}_{\text{3}}}{\text{CON}}{{\text{H}}_{\text{2}}}\)
S
With alcoholic KOH and \({\text{CHC}}{{\text{l}}_{\text{3}}}\) producesbad smell
324151
A compound of molecular formulae \({{\rm{C}}_{\rm{3}}}{{\rm{H}}_{\rm{6}}}{\rm{N}}\) shows following characteristics
(i) Get dissolved in acidic medium.
(ii) Does not react with benzoylchloride
(iii) Does not give carbylamine test
(iv) Does not evolute nitrogen gas on reacting with \(\mathrm{HNO}_{2}\)
The structure of the compound is
324150
The correct match of Column I (Amines) and Column II (Property) is
Column I
Column II
A
\({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{N}}{{\text{H}}_{\text{2}}}\)
P
Undergoes Lieberman nitrosoamine
B
\({{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}\)
Q
Undergoes Hoffmann bromamide reaction
C
\({{\text{(C}}{{\text{H}}_{\text{3}}}{\text{)}}_{\text{2}}}{\text{NH}}\)
R
Gives azo dye test
D
\({\text{C}}{{\text{H}}_{\text{3}}}{\text{CON}}{{\text{H}}_{\text{2}}}\)
S
With alcoholic KOH and \({\text{CHC}}{{\text{l}}_{\text{3}}}\) producesbad smell
324151
A compound of molecular formulae \({{\rm{C}}_{\rm{3}}}{{\rm{H}}_{\rm{6}}}{\rm{N}}\) shows following characteristics
(i) Get dissolved in acidic medium.
(ii) Does not react with benzoylchloride
(iii) Does not give carbylamine test
(iv) Does not evolute nitrogen gas on reacting with \(\mathrm{HNO}_{2}\)
The structure of the compound is
324150
The correct match of Column I (Amines) and Column II (Property) is
Column I
Column II
A
\({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{N}}{{\text{H}}_{\text{2}}}\)
P
Undergoes Lieberman nitrosoamine
B
\({{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}\)
Q
Undergoes Hoffmann bromamide reaction
C
\({{\text{(C}}{{\text{H}}_{\text{3}}}{\text{)}}_{\text{2}}}{\text{NH}}\)
R
Gives azo dye test
D
\({\text{C}}{{\text{H}}_{\text{3}}}{\text{CON}}{{\text{H}}_{\text{2}}}\)
S
With alcoholic KOH and \({\text{CHC}}{{\text{l}}_{\text{3}}}\) producesbad smell
324151
A compound of molecular formulae \({{\rm{C}}_{\rm{3}}}{{\rm{H}}_{\rm{6}}}{\rm{N}}\) shows following characteristics
(i) Get dissolved in acidic medium.
(ii) Does not react with benzoylchloride
(iii) Does not give carbylamine test
(iv) Does not evolute nitrogen gas on reacting with \(\mathrm{HNO}_{2}\)
The structure of the compound is