324125
Read Assertion and Reason carefully to mark the correct option given below.
Assertion :
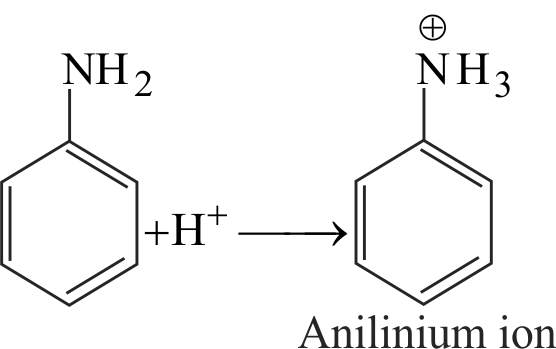
p-fluoroanilinium ion is more acidic than anilinium ion.
Reason :
Electron density in the N-H bond of p-fluoroanilinium ion decreases and release of a proton from p-fluoroanilinium ion is much easier than from anilinium ion.
324125
Read Assertion and Reason carefully to mark the correct option given below.
Assertion :
p-fluoroanilinium ion is more acidic than anilinium ion.
Reason :
Electron density in the N-H bond of p-fluoroanilinium ion decreases and release of a proton from p-fluoroanilinium ion is much easier than from anilinium ion.
324125
Read Assertion and Reason carefully to mark the correct option given below.
Assertion :
p-fluoroanilinium ion is more acidic than anilinium ion.
Reason :
Electron density in the N-H bond of p-fluoroanilinium ion decreases and release of a proton from p-fluoroanilinium ion is much easier than from anilinium ion.
324125
Read Assertion and Reason carefully to mark the correct option given below.
Assertion :
p-fluoroanilinium ion is more acidic than anilinium ion.
Reason :
Electron density in the N-H bond of p-fluoroanilinium ion decreases and release of a proton from p-fluoroanilinium ion is much easier than from anilinium ion.