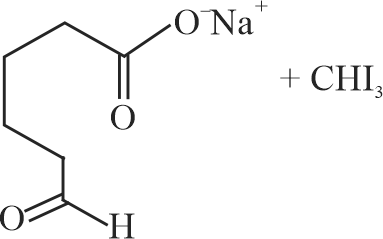
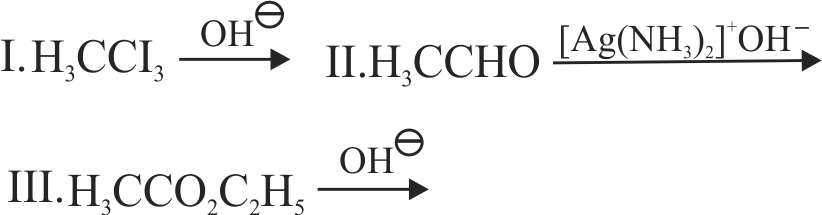323901
Several simple chemical tests are given to distinguish between the pair of compounds.
Which of the following pairs are correct?
(I) Propanal and propanone \({\mathrm{\rightarrow}}\) Silver mirror test
(II) Acetophenone and benzophenone \({\mathrm{\rightarrow}}\) Iodoform test
(III)Ethanal and propanal \({\mathrm{\rightarrow}}\) Fehling's test
(IV)Benzoic acid and ethylbenzoate \({\mathrm{\rightarrow}}\) Sodium bicarbonate test
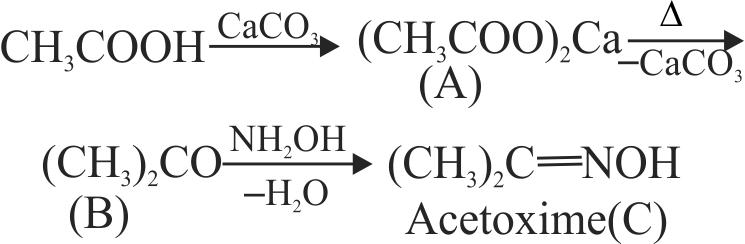
323902 A compound with molecular formula \(\mathrm{C}_{4} \mathrm{H}_{10} \mathrm{O}_{3}\) is converted by the action of acetyl chloride to a compound of molecular mass 190. The original compound \(\left(\mathrm{C}_{4} \mathrm{H}_{10} \mathrm{O}_{3}\right)\) has
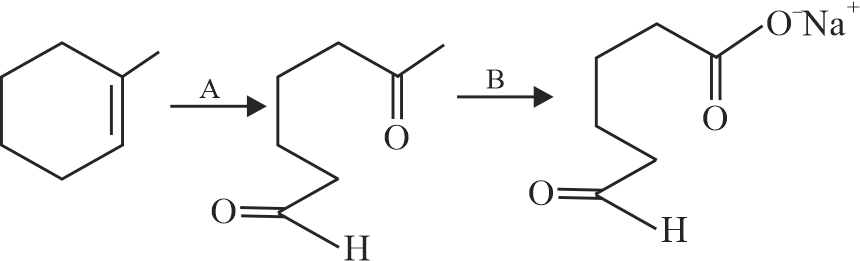
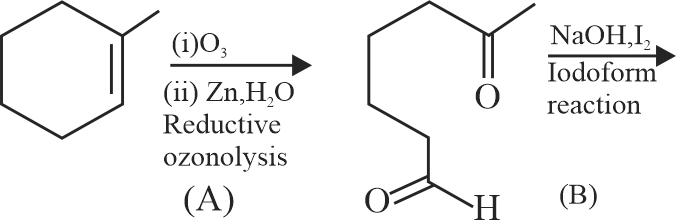
323903 An organic compound has an empirical formula \(\mathrm{C}_{5} \mathrm{H}_{10} \mathrm{O}\). This liquid gives a pale yellow precipitate on warming with iodine in alkaline KI solution. The resulting salt upon acidification gives an acid which easily undergoes decarboxylaction on mild heating. The structural formula of the organic liquid could be
323901
Several simple chemical tests are given to distinguish between the pair of compounds.
Which of the following pairs are correct?
(I) Propanal and propanone \({\mathrm{\rightarrow}}\) Silver mirror test
(II) Acetophenone and benzophenone \({\mathrm{\rightarrow}}\) Iodoform test
(III)Ethanal and propanal \({\mathrm{\rightarrow}}\) Fehling's test
(IV)Benzoic acid and ethylbenzoate \({\mathrm{\rightarrow}}\) Sodium bicarbonate test
323902 A compound with molecular formula \(\mathrm{C}_{4} \mathrm{H}_{10} \mathrm{O}_{3}\) is converted by the action of acetyl chloride to a compound of molecular mass 190. The original compound \(\left(\mathrm{C}_{4} \mathrm{H}_{10} \mathrm{O}_{3}\right)\) has
323903 An organic compound has an empirical formula \(\mathrm{C}_{5} \mathrm{H}_{10} \mathrm{O}\). This liquid gives a pale yellow precipitate on warming with iodine in alkaline KI solution. The resulting salt upon acidification gives an acid which easily undergoes decarboxylaction on mild heating. The structural formula of the organic liquid could be
323901
Several simple chemical tests are given to distinguish between the pair of compounds.
Which of the following pairs are correct?
(I) Propanal and propanone \({\mathrm{\rightarrow}}\) Silver mirror test
(II) Acetophenone and benzophenone \({\mathrm{\rightarrow}}\) Iodoform test
(III)Ethanal and propanal \({\mathrm{\rightarrow}}\) Fehling's test
(IV)Benzoic acid and ethylbenzoate \({\mathrm{\rightarrow}}\) Sodium bicarbonate test
323902 A compound with molecular formula \(\mathrm{C}_{4} \mathrm{H}_{10} \mathrm{O}_{3}\) is converted by the action of acetyl chloride to a compound of molecular mass 190. The original compound \(\left(\mathrm{C}_{4} \mathrm{H}_{10} \mathrm{O}_{3}\right)\) has
323903 An organic compound has an empirical formula \(\mathrm{C}_{5} \mathrm{H}_{10} \mathrm{O}\). This liquid gives a pale yellow precipitate on warming with iodine in alkaline KI solution. The resulting salt upon acidification gives an acid which easily undergoes decarboxylaction on mild heating. The structural formula of the organic liquid could be
323901
Several simple chemical tests are given to distinguish between the pair of compounds.
Which of the following pairs are correct?
(I) Propanal and propanone \({\mathrm{\rightarrow}}\) Silver mirror test
(II) Acetophenone and benzophenone \({\mathrm{\rightarrow}}\) Iodoform test
(III)Ethanal and propanal \({\mathrm{\rightarrow}}\) Fehling's test
(IV)Benzoic acid and ethylbenzoate \({\mathrm{\rightarrow}}\) Sodium bicarbonate test
323902 A compound with molecular formula \(\mathrm{C}_{4} \mathrm{H}_{10} \mathrm{O}_{3}\) is converted by the action of acetyl chloride to a compound of molecular mass 190. The original compound \(\left(\mathrm{C}_{4} \mathrm{H}_{10} \mathrm{O}_{3}\right)\) has
323903 An organic compound has an empirical formula \(\mathrm{C}_{5} \mathrm{H}_{10} \mathrm{O}\). This liquid gives a pale yellow precipitate on warming with iodine in alkaline KI solution. The resulting salt upon acidification gives an acid which easily undergoes decarboxylaction on mild heating. The structural formula of the organic liquid could be
323901
Several simple chemical tests are given to distinguish between the pair of compounds.
Which of the following pairs are correct?
(I) Propanal and propanone \({\mathrm{\rightarrow}}\) Silver mirror test
(II) Acetophenone and benzophenone \({\mathrm{\rightarrow}}\) Iodoform test
(III)Ethanal and propanal \({\mathrm{\rightarrow}}\) Fehling's test
(IV)Benzoic acid and ethylbenzoate \({\mathrm{\rightarrow}}\) Sodium bicarbonate test
323902 A compound with molecular formula \(\mathrm{C}_{4} \mathrm{H}_{10} \mathrm{O}_{3}\) is converted by the action of acetyl chloride to a compound of molecular mass 190. The original compound \(\left(\mathrm{C}_{4} \mathrm{H}_{10} \mathrm{O}_{3}\right)\) has
323903 An organic compound has an empirical formula \(\mathrm{C}_{5} \mathrm{H}_{10} \mathrm{O}\). This liquid gives a pale yellow precipitate on warming with iodine in alkaline KI solution. The resulting salt upon acidification gives an acid which easily undergoes decarboxylaction on mild heating. The structural formula of the organic liquid could be