323876
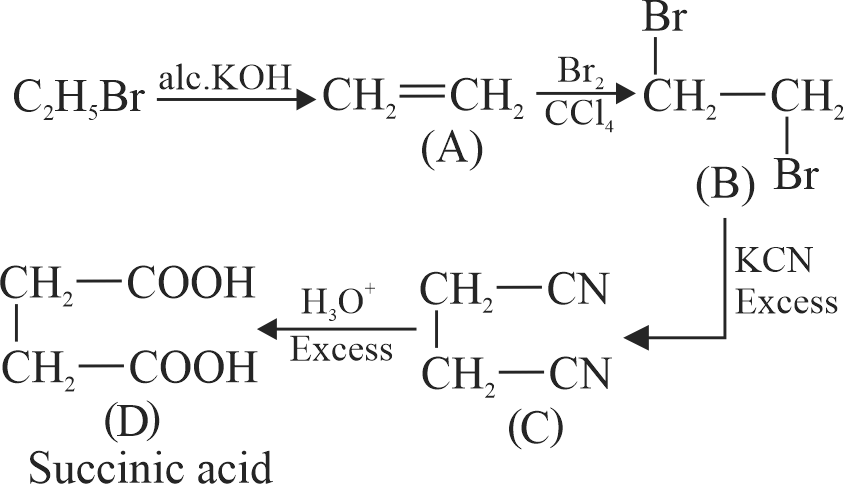
\({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{Br}}\xrightarrow{{{\text{alc}}{\text{.KOH}}}}{\text{A}}\xrightarrow[{{\text{CC}}{{\text{l}}_{\text{4}}}}]{{{\text{B}}{{\text{r}}_{\text{2}}}}}{\text{B}}\xrightarrow[{{\text{Excess}}}]{{{\text{KCN}}}}\)
\({\text{C}}\xrightarrow[{{\text{Excess}}}]{{{{\text{H}}_{\text{3}}}{{\text{O}}^{\text{ + }}}}}{\text{D}}\)
Acid D formed in above reaction is
323876
\({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{Br}}\xrightarrow{{{\text{alc}}{\text{.KOH}}}}{\text{A}}\xrightarrow[{{\text{CC}}{{\text{l}}_{\text{4}}}}]{{{\text{B}}{{\text{r}}_{\text{2}}}}}{\text{B}}\xrightarrow[{{\text{Excess}}}]{{{\text{KCN}}}}\)
\({\text{C}}\xrightarrow[{{\text{Excess}}}]{{{{\text{H}}_{\text{3}}}{{\text{O}}^{\text{ + }}}}}{\text{D}}\)
Acid D formed in above reaction is
323876
\({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{Br}}\xrightarrow{{{\text{alc}}{\text{.KOH}}}}{\text{A}}\xrightarrow[{{\text{CC}}{{\text{l}}_{\text{4}}}}]{{{\text{B}}{{\text{r}}_{\text{2}}}}}{\text{B}}\xrightarrow[{{\text{Excess}}}]{{{\text{KCN}}}}\)
\({\text{C}}\xrightarrow[{{\text{Excess}}}]{{{{\text{H}}_{\text{3}}}{{\text{O}}^{\text{ + }}}}}{\text{D}}\)
Acid D formed in above reaction is
323876
\({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{Br}}\xrightarrow{{{\text{alc}}{\text{.KOH}}}}{\text{A}}\xrightarrow[{{\text{CC}}{{\text{l}}_{\text{4}}}}]{{{\text{B}}{{\text{r}}_{\text{2}}}}}{\text{B}}\xrightarrow[{{\text{Excess}}}]{{{\text{KCN}}}}\)
\({\text{C}}\xrightarrow[{{\text{Excess}}}]{{{{\text{H}}_{\text{3}}}{{\text{O}}^{\text{ + }}}}}{\text{D}}\)
Acid D formed in above reaction is
323876
\({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{Br}}\xrightarrow{{{\text{alc}}{\text{.KOH}}}}{\text{A}}\xrightarrow[{{\text{CC}}{{\text{l}}_{\text{4}}}}]{{{\text{B}}{{\text{r}}_{\text{2}}}}}{\text{B}}\xrightarrow[{{\text{Excess}}}]{{{\text{KCN}}}}\)
\({\text{C}}\xrightarrow[{{\text{Excess}}}]{{{{\text{H}}_{\text{3}}}{{\text{O}}^{\text{ + }}}}}{\text{D}}\)
Acid D formed in above reaction is