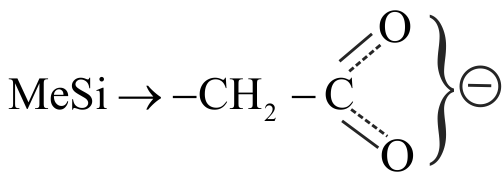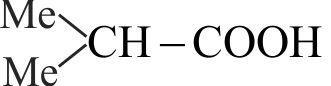323698
Assertion :
\({\text{m}}\) -Chlorobenzoic acid is a stronger acid than p-chlorobenzoic acid.
Reason :
In \(\mathrm{m}\)-chlorobenzoic acid both -I effect and \(+\mathrm{R}\) effect of \(\mathrm{Cl}\) operate but in \(\mathrm{p-}\) chlorobenzoic acid only \(+\mathrm{R}\) effect of \(\mathrm{Cl}\) operates.
323698
Assertion :
\({\text{m}}\) -Chlorobenzoic acid is a stronger acid than p-chlorobenzoic acid.
Reason :
In \(\mathrm{m}\)-chlorobenzoic acid both -I effect and \(+\mathrm{R}\) effect of \(\mathrm{Cl}\) operate but in \(\mathrm{p-}\) chlorobenzoic acid only \(+\mathrm{R}\) effect of \(\mathrm{Cl}\) operates.
323698
Assertion :
\({\text{m}}\) -Chlorobenzoic acid is a stronger acid than p-chlorobenzoic acid.
Reason :
In \(\mathrm{m}\)-chlorobenzoic acid both -I effect and \(+\mathrm{R}\) effect of \(\mathrm{Cl}\) operate but in \(\mathrm{p-}\) chlorobenzoic acid only \(+\mathrm{R}\) effect of \(\mathrm{Cl}\) operates.
323698
Assertion :
\({\text{m}}\) -Chlorobenzoic acid is a stronger acid than p-chlorobenzoic acid.
Reason :
In \(\mathrm{m}\)-chlorobenzoic acid both -I effect and \(+\mathrm{R}\) effect of \(\mathrm{Cl}\) operate but in \(\mathrm{p-}\) chlorobenzoic acid only \(+\mathrm{R}\) effect of \(\mathrm{Cl}\) operates.