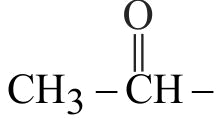323658
Amongst the following the total number of compounds that gives positive iodoform test are ____ .
\({\mathrm{\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{OH}, \quad \mathrm{PhCHO}, \quad \mathrm{CH}_{3} \mathrm{COCH}_{3}}}\), \({\mathrm{\left(\mathrm{CH}_{3}\right)_{2} \mathrm{CHOH}, \quad \mathrm{HCHO}, \quad \mathrm{CH}_{3}\left(\mathrm{CH}_{2}\right)_{2} \mathrm{COCH}_{3}}}\), \({\mathrm{\mathrm{PhCH}_{2} \mathrm{OH}, \mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{COPh}}}\)
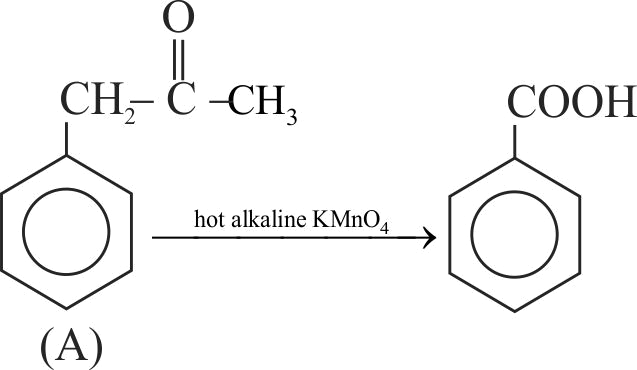
323661 Compound (A), \(\mathrm{C}_{9} \mathrm{H}_{10} \mathrm{O}\), is inert to \(\mathrm{Br}_{2}\) in \(\mathrm{CCl}_{4}\). Vigorous oxidation with hot alkaline \(\mathrm{KMnO}_{4}\) yields benzoic acid. Compound "A" gives red precipitate with 2,4-dinitrophenylhydrazine and yellow with \(\mathrm{NaOI}\). The possible structure of compound (A) will be :
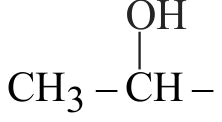
323662 An organic liquid has an empirical formula \(\mathrm{C}_{4} \mathrm{H}_{8} \mathrm{O}\). The liquid gives a pale yellow precipitate on warming with iodine in alkaline potassium hydroxide solution. The structural formula of the organic liquid could be
323658
Amongst the following the total number of compounds that gives positive iodoform test are ____ .
\({\mathrm{\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{OH}, \quad \mathrm{PhCHO}, \quad \mathrm{CH}_{3} \mathrm{COCH}_{3}}}\), \({\mathrm{\left(\mathrm{CH}_{3}\right)_{2} \mathrm{CHOH}, \quad \mathrm{HCHO}, \quad \mathrm{CH}_{3}\left(\mathrm{CH}_{2}\right)_{2} \mathrm{COCH}_{3}}}\), \({\mathrm{\mathrm{PhCH}_{2} \mathrm{OH}, \mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{COPh}}}\)
323661 Compound (A), \(\mathrm{C}_{9} \mathrm{H}_{10} \mathrm{O}\), is inert to \(\mathrm{Br}_{2}\) in \(\mathrm{CCl}_{4}\). Vigorous oxidation with hot alkaline \(\mathrm{KMnO}_{4}\) yields benzoic acid. Compound "A" gives red precipitate with 2,4-dinitrophenylhydrazine and yellow with \(\mathrm{NaOI}\). The possible structure of compound (A) will be :
323662 An organic liquid has an empirical formula \(\mathrm{C}_{4} \mathrm{H}_{8} \mathrm{O}\). The liquid gives a pale yellow precipitate on warming with iodine in alkaline potassium hydroxide solution. The structural formula of the organic liquid could be
323658
Amongst the following the total number of compounds that gives positive iodoform test are ____ .
\({\mathrm{\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{OH}, \quad \mathrm{PhCHO}, \quad \mathrm{CH}_{3} \mathrm{COCH}_{3}}}\), \({\mathrm{\left(\mathrm{CH}_{3}\right)_{2} \mathrm{CHOH}, \quad \mathrm{HCHO}, \quad \mathrm{CH}_{3}\left(\mathrm{CH}_{2}\right)_{2} \mathrm{COCH}_{3}}}\), \({\mathrm{\mathrm{PhCH}_{2} \mathrm{OH}, \mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{COPh}}}\)
323661 Compound (A), \(\mathrm{C}_{9} \mathrm{H}_{10} \mathrm{O}\), is inert to \(\mathrm{Br}_{2}\) in \(\mathrm{CCl}_{4}\). Vigorous oxidation with hot alkaline \(\mathrm{KMnO}_{4}\) yields benzoic acid. Compound "A" gives red precipitate with 2,4-dinitrophenylhydrazine and yellow with \(\mathrm{NaOI}\). The possible structure of compound (A) will be :
323662 An organic liquid has an empirical formula \(\mathrm{C}_{4} \mathrm{H}_{8} \mathrm{O}\). The liquid gives a pale yellow precipitate on warming with iodine in alkaline potassium hydroxide solution. The structural formula of the organic liquid could be
323658
Amongst the following the total number of compounds that gives positive iodoform test are ____ .
\({\mathrm{\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{OH}, \quad \mathrm{PhCHO}, \quad \mathrm{CH}_{3} \mathrm{COCH}_{3}}}\), \({\mathrm{\left(\mathrm{CH}_{3}\right)_{2} \mathrm{CHOH}, \quad \mathrm{HCHO}, \quad \mathrm{CH}_{3}\left(\mathrm{CH}_{2}\right)_{2} \mathrm{COCH}_{3}}}\), \({\mathrm{\mathrm{PhCH}_{2} \mathrm{OH}, \mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{COPh}}}\)
323661 Compound (A), \(\mathrm{C}_{9} \mathrm{H}_{10} \mathrm{O}\), is inert to \(\mathrm{Br}_{2}\) in \(\mathrm{CCl}_{4}\). Vigorous oxidation with hot alkaline \(\mathrm{KMnO}_{4}\) yields benzoic acid. Compound "A" gives red precipitate with 2,4-dinitrophenylhydrazine and yellow with \(\mathrm{NaOI}\). The possible structure of compound (A) will be :
323662 An organic liquid has an empirical formula \(\mathrm{C}_{4} \mathrm{H}_{8} \mathrm{O}\). The liquid gives a pale yellow precipitate on warming with iodine in alkaline potassium hydroxide solution. The structural formula of the organic liquid could be
323658
Amongst the following the total number of compounds that gives positive iodoform test are ____ .
\({\mathrm{\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{OH}, \quad \mathrm{PhCHO}, \quad \mathrm{CH}_{3} \mathrm{COCH}_{3}}}\), \({\mathrm{\left(\mathrm{CH}_{3}\right)_{2} \mathrm{CHOH}, \quad \mathrm{HCHO}, \quad \mathrm{CH}_{3}\left(\mathrm{CH}_{2}\right)_{2} \mathrm{COCH}_{3}}}\), \({\mathrm{\mathrm{PhCH}_{2} \mathrm{OH}, \mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{COPh}}}\)
323661 Compound (A), \(\mathrm{C}_{9} \mathrm{H}_{10} \mathrm{O}\), is inert to \(\mathrm{Br}_{2}\) in \(\mathrm{CCl}_{4}\). Vigorous oxidation with hot alkaline \(\mathrm{KMnO}_{4}\) yields benzoic acid. Compound "A" gives red precipitate with 2,4-dinitrophenylhydrazine and yellow with \(\mathrm{NaOI}\). The possible structure of compound (A) will be :
323662 An organic liquid has an empirical formula \(\mathrm{C}_{4} \mathrm{H}_{8} \mathrm{O}\). The liquid gives a pale yellow precipitate on warming with iodine in alkaline potassium hydroxide solution. The structural formula of the organic liquid could be