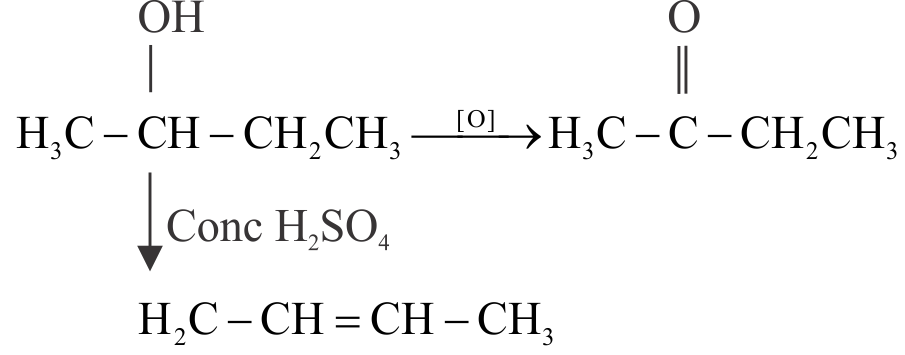323598 A substance \(\mathrm{C}_{4} \mathrm{H}_{10} \mathrm{O}\) on oxidation yields compound \(\mathrm{C}_{4} \mathrm{H}_{8} \mathrm{O}\) which gives an oxime and a positive iodoform test. The original substance on treatment with conc. \(\mathrm{H}_{2} \mathrm{SO}_{4}\) gives \(\mathrm{C}_{4} \mathrm{H}_{8}\). The compound is
323598 A substance \(\mathrm{C}_{4} \mathrm{H}_{10} \mathrm{O}\) on oxidation yields compound \(\mathrm{C}_{4} \mathrm{H}_{8} \mathrm{O}\) which gives an oxime and a positive iodoform test. The original substance on treatment with conc. \(\mathrm{H}_{2} \mathrm{SO}_{4}\) gives \(\mathrm{C}_{4} \mathrm{H}_{8}\). The compound is
323598 A substance \(\mathrm{C}_{4} \mathrm{H}_{10} \mathrm{O}\) on oxidation yields compound \(\mathrm{C}_{4} \mathrm{H}_{8} \mathrm{O}\) which gives an oxime and a positive iodoform test. The original substance on treatment with conc. \(\mathrm{H}_{2} \mathrm{SO}_{4}\) gives \(\mathrm{C}_{4} \mathrm{H}_{8}\). The compound is
323598 A substance \(\mathrm{C}_{4} \mathrm{H}_{10} \mathrm{O}\) on oxidation yields compound \(\mathrm{C}_{4} \mathrm{H}_{8} \mathrm{O}\) which gives an oxime and a positive iodoform test. The original substance on treatment with conc. \(\mathrm{H}_{2} \mathrm{SO}_{4}\) gives \(\mathrm{C}_{4} \mathrm{H}_{8}\). The compound is
323598 A substance \(\mathrm{C}_{4} \mathrm{H}_{10} \mathrm{O}\) on oxidation yields compound \(\mathrm{C}_{4} \mathrm{H}_{8} \mathrm{O}\) which gives an oxime and a positive iodoform test. The original substance on treatment with conc. \(\mathrm{H}_{2} \mathrm{SO}_{4}\) gives \(\mathrm{C}_{4} \mathrm{H}_{8}\). The compound is