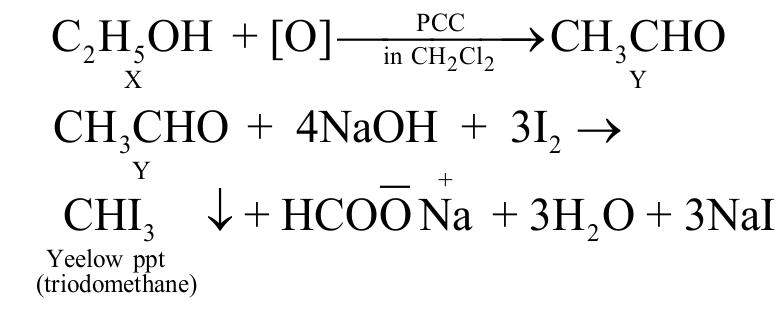
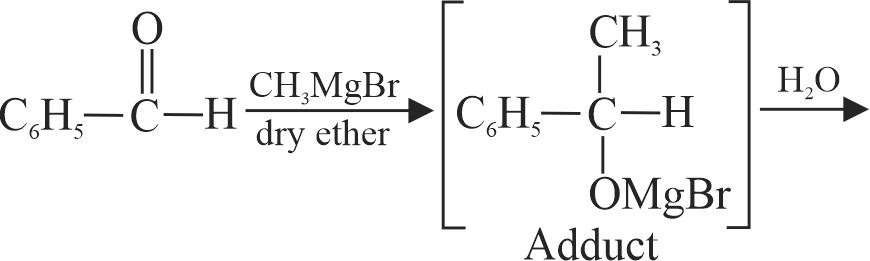
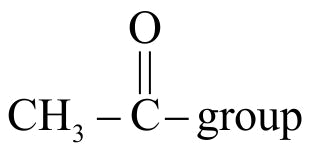
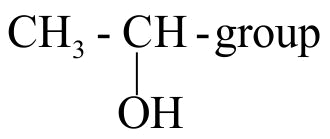
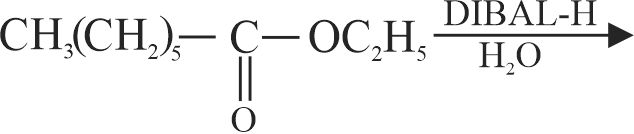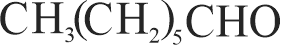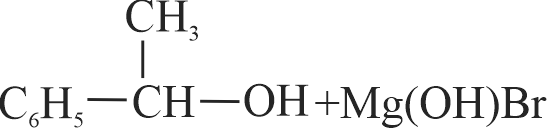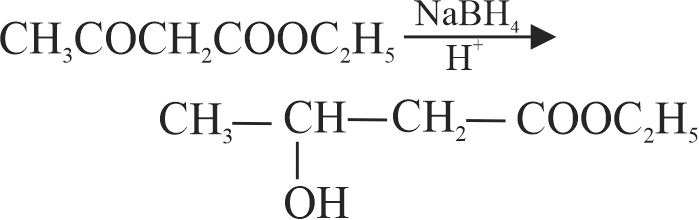323568 An organic compound \(\mathrm{X}\) on treatment with pyridinium chloro chromate in dichloromethane gives compound \(\mathrm{Y}\). Compound \(\mathrm{Y}\) reacts with \(\mathrm{I}_{2}\) and alkali to form triiodomethane. The compound \(\mathrm{X}\) is
323569 A compound \([\mathrm{A}]\) has the molecular formula \(\mathrm{C}_{2} \mathrm{Cl}_{3} \mathrm{OH}\). It reduces Fehling's solution and on oxidation gives a monocarboxylic acid [B]. [A] is obtained by the action of \(\mathrm{Cl}_{2}\) on ethyl alcohol. [A] is
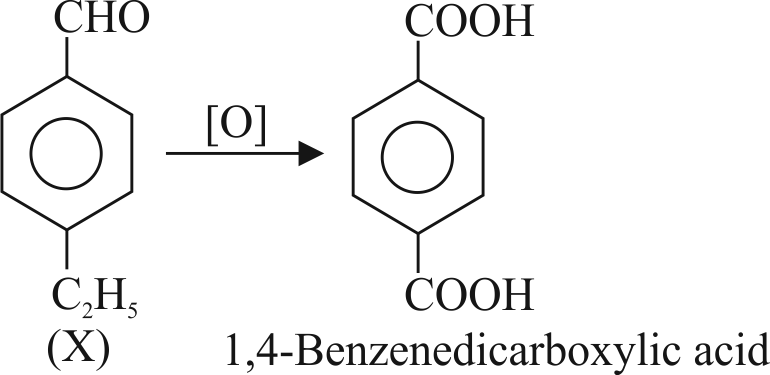
323571 An organic compound \({\text{X}}\) with molecular formula \(\mathrm{C}_{9} \mathrm{H}_{10} \mathrm{O}\) gives positive 2,4 - DNP and Tollen's test. It undergoes Cannizarro reaction and on vigorous oxidation, it gives 1,4 Benzenedicarboylic acid. Compound \((\mathrm{X})\) is:
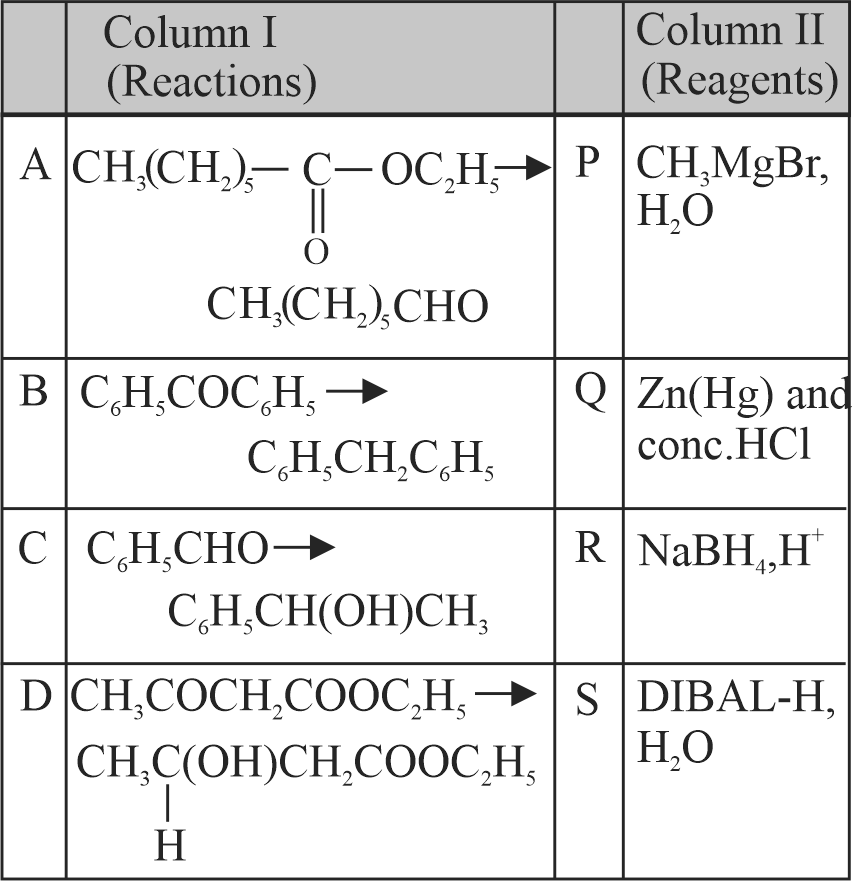
323572 A substance \(\mathrm{C}_{4} \mathrm{H}_{10} \mathrm{O}\) yields on oxidation a compound, \(\mathrm{C}_{4} \mathrm{H}_{8} \mathrm{O}\) which gives an oxime and a positive iodoform test. The original substance on treatment with conc. \(\mathrm{H}_{2} \mathrm{SO}_{4}\) gives \(\mathrm{C}_{4} \mathrm{H}_{8}\). The structure of the compound is
323568 An organic compound \(\mathrm{X}\) on treatment with pyridinium chloro chromate in dichloromethane gives compound \(\mathrm{Y}\). Compound \(\mathrm{Y}\) reacts with \(\mathrm{I}_{2}\) and alkali to form triiodomethane. The compound \(\mathrm{X}\) is
323569 A compound \([\mathrm{A}]\) has the molecular formula \(\mathrm{C}_{2} \mathrm{Cl}_{3} \mathrm{OH}\). It reduces Fehling's solution and on oxidation gives a monocarboxylic acid [B]. [A] is obtained by the action of \(\mathrm{Cl}_{2}\) on ethyl alcohol. [A] is
323571 An organic compound \({\text{X}}\) with molecular formula \(\mathrm{C}_{9} \mathrm{H}_{10} \mathrm{O}\) gives positive 2,4 - DNP and Tollen's test. It undergoes Cannizarro reaction and on vigorous oxidation, it gives 1,4 Benzenedicarboylic acid. Compound \((\mathrm{X})\) is:
323572 A substance \(\mathrm{C}_{4} \mathrm{H}_{10} \mathrm{O}\) yields on oxidation a compound, \(\mathrm{C}_{4} \mathrm{H}_{8} \mathrm{O}\) which gives an oxime and a positive iodoform test. The original substance on treatment with conc. \(\mathrm{H}_{2} \mathrm{SO}_{4}\) gives \(\mathrm{C}_{4} \mathrm{H}_{8}\). The structure of the compound is
323568 An organic compound \(\mathrm{X}\) on treatment with pyridinium chloro chromate in dichloromethane gives compound \(\mathrm{Y}\). Compound \(\mathrm{Y}\) reacts with \(\mathrm{I}_{2}\) and alkali to form triiodomethane. The compound \(\mathrm{X}\) is
323569 A compound \([\mathrm{A}]\) has the molecular formula \(\mathrm{C}_{2} \mathrm{Cl}_{3} \mathrm{OH}\). It reduces Fehling's solution and on oxidation gives a monocarboxylic acid [B]. [A] is obtained by the action of \(\mathrm{Cl}_{2}\) on ethyl alcohol. [A] is
323571 An organic compound \({\text{X}}\) with molecular formula \(\mathrm{C}_{9} \mathrm{H}_{10} \mathrm{O}\) gives positive 2,4 - DNP and Tollen's test. It undergoes Cannizarro reaction and on vigorous oxidation, it gives 1,4 Benzenedicarboylic acid. Compound \((\mathrm{X})\) is:
323572 A substance \(\mathrm{C}_{4} \mathrm{H}_{10} \mathrm{O}\) yields on oxidation a compound, \(\mathrm{C}_{4} \mathrm{H}_{8} \mathrm{O}\) which gives an oxime and a positive iodoform test. The original substance on treatment with conc. \(\mathrm{H}_{2} \mathrm{SO}_{4}\) gives \(\mathrm{C}_{4} \mathrm{H}_{8}\). The structure of the compound is
323568 An organic compound \(\mathrm{X}\) on treatment with pyridinium chloro chromate in dichloromethane gives compound \(\mathrm{Y}\). Compound \(\mathrm{Y}\) reacts with \(\mathrm{I}_{2}\) and alkali to form triiodomethane. The compound \(\mathrm{X}\) is
323569 A compound \([\mathrm{A}]\) has the molecular formula \(\mathrm{C}_{2} \mathrm{Cl}_{3} \mathrm{OH}\). It reduces Fehling's solution and on oxidation gives a monocarboxylic acid [B]. [A] is obtained by the action of \(\mathrm{Cl}_{2}\) on ethyl alcohol. [A] is
323571 An organic compound \({\text{X}}\) with molecular formula \(\mathrm{C}_{9} \mathrm{H}_{10} \mathrm{O}\) gives positive 2,4 - DNP and Tollen's test. It undergoes Cannizarro reaction and on vigorous oxidation, it gives 1,4 Benzenedicarboylic acid. Compound \((\mathrm{X})\) is:
323572 A substance \(\mathrm{C}_{4} \mathrm{H}_{10} \mathrm{O}\) yields on oxidation a compound, \(\mathrm{C}_{4} \mathrm{H}_{8} \mathrm{O}\) which gives an oxime and a positive iodoform test. The original substance on treatment with conc. \(\mathrm{H}_{2} \mathrm{SO}_{4}\) gives \(\mathrm{C}_{4} \mathrm{H}_{8}\). The structure of the compound is
323568 An organic compound \(\mathrm{X}\) on treatment with pyridinium chloro chromate in dichloromethane gives compound \(\mathrm{Y}\). Compound \(\mathrm{Y}\) reacts with \(\mathrm{I}_{2}\) and alkali to form triiodomethane. The compound \(\mathrm{X}\) is
323569 A compound \([\mathrm{A}]\) has the molecular formula \(\mathrm{C}_{2} \mathrm{Cl}_{3} \mathrm{OH}\). It reduces Fehling's solution and on oxidation gives a monocarboxylic acid [B]. [A] is obtained by the action of \(\mathrm{Cl}_{2}\) on ethyl alcohol. [A] is
323571 An organic compound \({\text{X}}\) with molecular formula \(\mathrm{C}_{9} \mathrm{H}_{10} \mathrm{O}\) gives positive 2,4 - DNP and Tollen's test. It undergoes Cannizarro reaction and on vigorous oxidation, it gives 1,4 Benzenedicarboylic acid. Compound \((\mathrm{X})\) is:
323572 A substance \(\mathrm{C}_{4} \mathrm{H}_{10} \mathrm{O}\) yields on oxidation a compound, \(\mathrm{C}_{4} \mathrm{H}_{8} \mathrm{O}\) which gives an oxime and a positive iodoform test. The original substance on treatment with conc. \(\mathrm{H}_{2} \mathrm{SO}_{4}\) gives \(\mathrm{C}_{4} \mathrm{H}_{8}\). The structure of the compound is