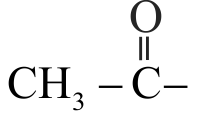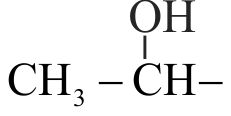323432
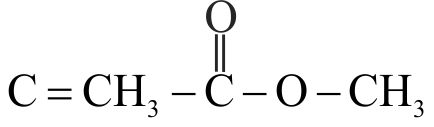
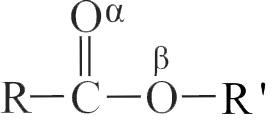
Identify the functional group in compound \(\mathrm{C}\) in the following reaction.
\({\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{ - Br}}\) \(\xrightarrow{{{\text{ Mg}}\,\,{\text{/}}\,\,{\text{ether }}}}{\text{(A)}}\) \(\xrightarrow[{{\text{(ii) Water}}}]{{{\text{(i) C}}{{\text{O}}_{\text{2}}}}}{\text{(B)}}\) \(\xrightarrow[\Delta ]{{{\text{C}}{{\text{H}}_{\text{3}}}{\text{OH,}}{{\text{H}}^{{\text{ + }}}}}}{\text{(C)}}\)
323434
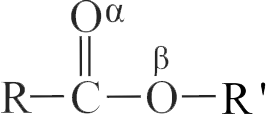
Which \(\mathrm{H}\) atom in the following ester is most acidic?
\(\begin{array}{*{20}{c}}{{\rm{C}}{{\rm{H}}_{\rm{3}}}}&{{\rm{C}}{{\rm{H}}_{\rm{2}}}}&{{\rm{COC}}{{\rm{H}}_{\rm{2}}}}&{{\rm{COO}}}&{{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{C}}{{\rm{H}}_{\rm{3}}}}\\{\rm{1}}&{\rm{2}}&{\rm{3}}&{}&{{\rm{4}}\,\,\,\,\,\,\,\,{\rm{5}}}\end{array}\)
323432
Identify the functional group in compound \(\mathrm{C}\) in the following reaction.
\({\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{ - Br}}\) \(\xrightarrow{{{\text{ Mg}}\,\,{\text{/}}\,\,{\text{ether }}}}{\text{(A)}}\) \(\xrightarrow[{{\text{(ii) Water}}}]{{{\text{(i) C}}{{\text{O}}_{\text{2}}}}}{\text{(B)}}\) \(\xrightarrow[\Delta ]{{{\text{C}}{{\text{H}}_{\text{3}}}{\text{OH,}}{{\text{H}}^{{\text{ + }}}}}}{\text{(C)}}\)
323434
Which \(\mathrm{H}\) atom in the following ester is most acidic?
\(\begin{array}{*{20}{c}}{{\rm{C}}{{\rm{H}}_{\rm{3}}}}&{{\rm{C}}{{\rm{H}}_{\rm{2}}}}&{{\rm{COC}}{{\rm{H}}_{\rm{2}}}}&{{\rm{COO}}}&{{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{C}}{{\rm{H}}_{\rm{3}}}}\\{\rm{1}}&{\rm{2}}&{\rm{3}}&{}&{{\rm{4}}\,\,\,\,\,\,\,\,{\rm{5}}}\end{array}\)
323432
Identify the functional group in compound \(\mathrm{C}\) in the following reaction.
\({\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{ - Br}}\) \(\xrightarrow{{{\text{ Mg}}\,\,{\text{/}}\,\,{\text{ether }}}}{\text{(A)}}\) \(\xrightarrow[{{\text{(ii) Water}}}]{{{\text{(i) C}}{{\text{O}}_{\text{2}}}}}{\text{(B)}}\) \(\xrightarrow[\Delta ]{{{\text{C}}{{\text{H}}_{\text{3}}}{\text{OH,}}{{\text{H}}^{{\text{ + }}}}}}{\text{(C)}}\)
323434
Which \(\mathrm{H}\) atom in the following ester is most acidic?
\(\begin{array}{*{20}{c}}{{\rm{C}}{{\rm{H}}_{\rm{3}}}}&{{\rm{C}}{{\rm{H}}_{\rm{2}}}}&{{\rm{COC}}{{\rm{H}}_{\rm{2}}}}&{{\rm{COO}}}&{{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{C}}{{\rm{H}}_{\rm{3}}}}\\{\rm{1}}&{\rm{2}}&{\rm{3}}&{}&{{\rm{4}}\,\,\,\,\,\,\,\,{\rm{5}}}\end{array}\)
323432
Identify the functional group in compound \(\mathrm{C}\) in the following reaction.
\({\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{ - Br}}\) \(\xrightarrow{{{\text{ Mg}}\,\,{\text{/}}\,\,{\text{ether }}}}{\text{(A)}}\) \(\xrightarrow[{{\text{(ii) Water}}}]{{{\text{(i) C}}{{\text{O}}_{\text{2}}}}}{\text{(B)}}\) \(\xrightarrow[\Delta ]{{{\text{C}}{{\text{H}}_{\text{3}}}{\text{OH,}}{{\text{H}}^{{\text{ + }}}}}}{\text{(C)}}\)
323434
Which \(\mathrm{H}\) atom in the following ester is most acidic?
\(\begin{array}{*{20}{c}}{{\rm{C}}{{\rm{H}}_{\rm{3}}}}&{{\rm{C}}{{\rm{H}}_{\rm{2}}}}&{{\rm{COC}}{{\rm{H}}_{\rm{2}}}}&{{\rm{COO}}}&{{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{C}}{{\rm{H}}_{\rm{3}}}}\\{\rm{1}}&{\rm{2}}&{\rm{3}}&{}&{{\rm{4}}\,\,\,\,\,\,\,\,{\rm{5}}}\end{array}\)