323298
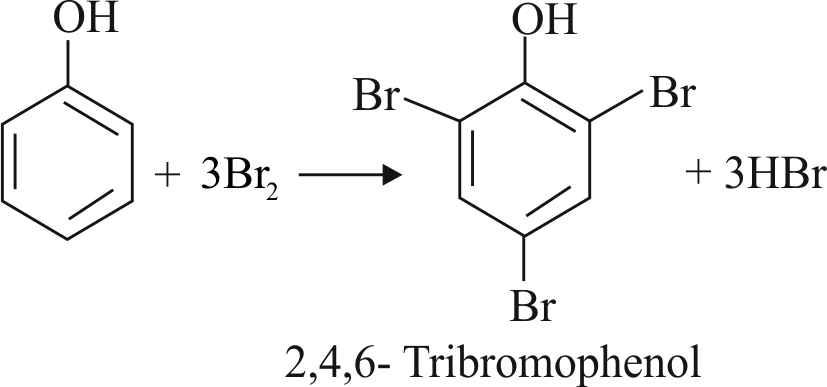
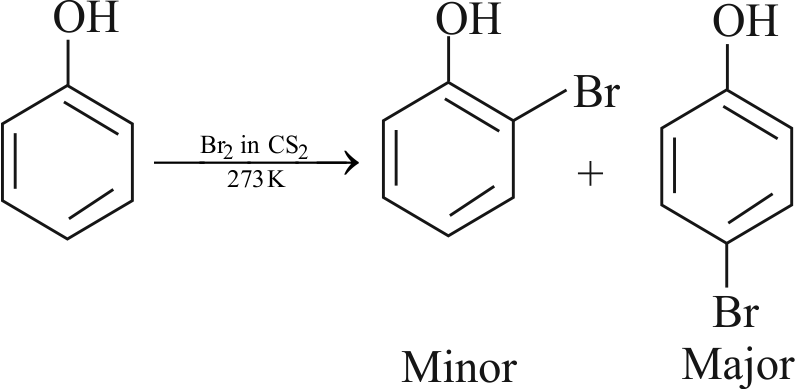
Assertion :
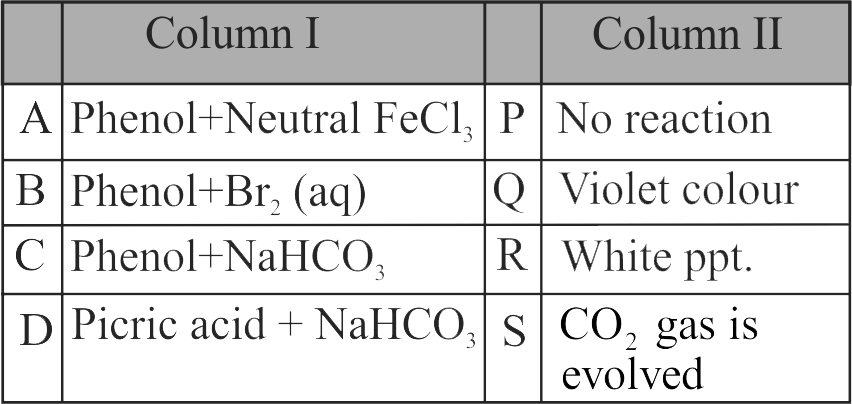
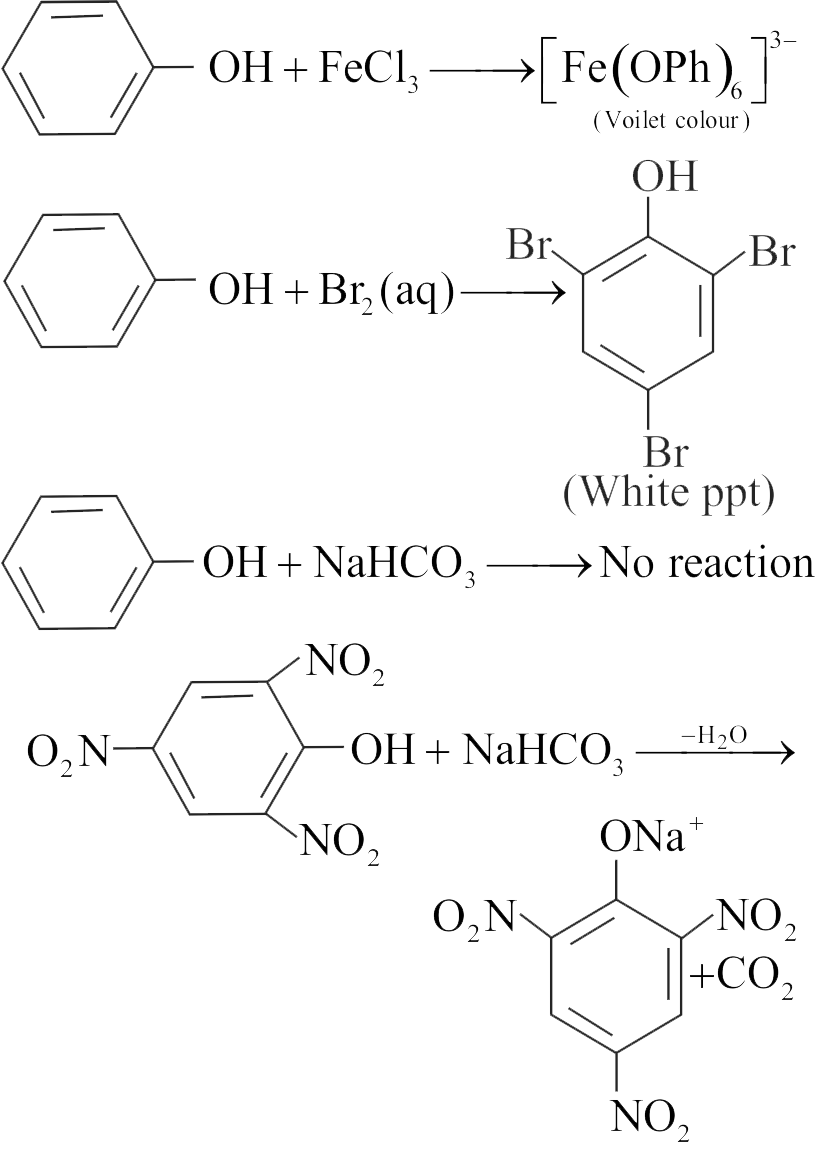
With \(\mathrm{Br}_{2}-\mathrm{H}_{2} \mathrm{O}\), phenol gives \(2,4,6-\) tribromophenol but with \(\mathrm{Br}_{2}-\mathrm{CS}_{2}\), it gives 4-bromophenol as the major product.
Reason :
In water, ionisation of phenol is enhanced but in \(\mathrm{CS}_{2}\), it is greatly suppressed.
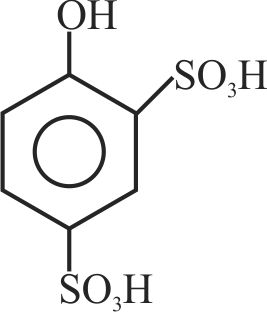
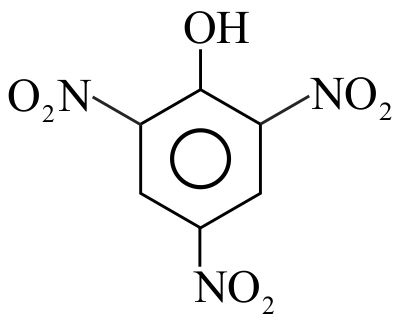
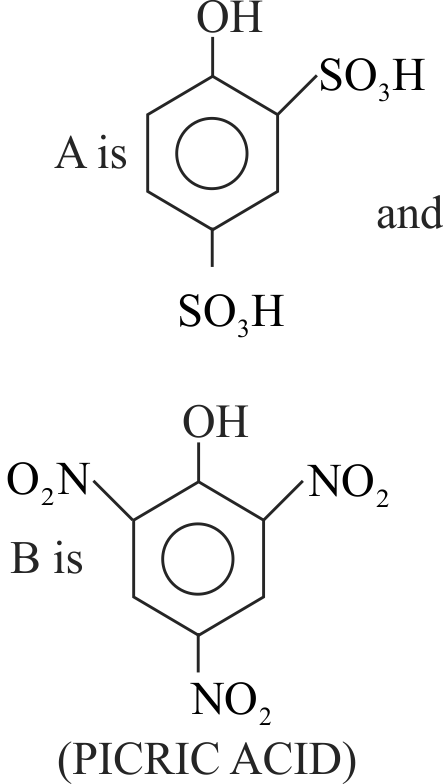
323299 \({\text{Phenol }}\xrightarrow{{{\text{ conc}}{\text{. }}{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}}}{\text{A}}\xrightarrow{{{\text{ conc}}{\text{. HN}}{{\text{O}}_{\text{3}}}}}{\text{B}}\). Here \({\text{A}}\) and \(\mathrm{B}\) are respectively:
323298
Assertion :
With \(\mathrm{Br}_{2}-\mathrm{H}_{2} \mathrm{O}\), phenol gives \(2,4,6-\) tribromophenol but with \(\mathrm{Br}_{2}-\mathrm{CS}_{2}\), it gives 4-bromophenol as the major product.
Reason :
In water, ionisation of phenol is enhanced but in \(\mathrm{CS}_{2}\), it is greatly suppressed.
323299 \({\text{Phenol }}\xrightarrow{{{\text{ conc}}{\text{. }}{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}}}{\text{A}}\xrightarrow{{{\text{ conc}}{\text{. HN}}{{\text{O}}_{\text{3}}}}}{\text{B}}\). Here \({\text{A}}\) and \(\mathrm{B}\) are respectively:
323298
Assertion :
With \(\mathrm{Br}_{2}-\mathrm{H}_{2} \mathrm{O}\), phenol gives \(2,4,6-\) tribromophenol but with \(\mathrm{Br}_{2}-\mathrm{CS}_{2}\), it gives 4-bromophenol as the major product.
Reason :
In water, ionisation of phenol is enhanced but in \(\mathrm{CS}_{2}\), it is greatly suppressed.
323299 \({\text{Phenol }}\xrightarrow{{{\text{ conc}}{\text{. }}{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}}}{\text{A}}\xrightarrow{{{\text{ conc}}{\text{. HN}}{{\text{O}}_{\text{3}}}}}{\text{B}}\). Here \({\text{A}}\) and \(\mathrm{B}\) are respectively:
323298
Assertion :
With \(\mathrm{Br}_{2}-\mathrm{H}_{2} \mathrm{O}\), phenol gives \(2,4,6-\) tribromophenol but with \(\mathrm{Br}_{2}-\mathrm{CS}_{2}\), it gives 4-bromophenol as the major product.
Reason :
In water, ionisation of phenol is enhanced but in \(\mathrm{CS}_{2}\), it is greatly suppressed.
323299 \({\text{Phenol }}\xrightarrow{{{\text{ conc}}{\text{. }}{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}}}{\text{A}}\xrightarrow{{{\text{ conc}}{\text{. HN}}{{\text{O}}_{\text{3}}}}}{\text{B}}\). Here \({\text{A}}\) and \(\mathrm{B}\) are respectively: