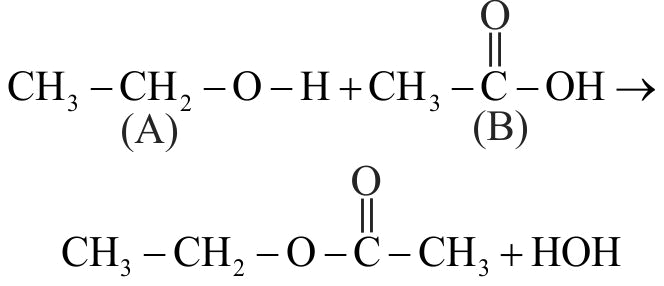
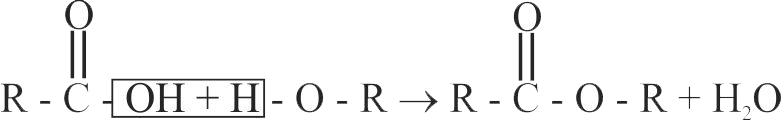323203 An organic compound ' \({\text{A}}\) ' containing \({\text{C, H}}\) and \({\text{O}}\) has a pleasant odour with boiling point \(78^{\circ} \mathrm{C}\). On boiling 'A' with conc \(\mathrm{H}_{2} \mathrm{SO}_{4}\), a colourless gas is released. The organic liquid ' \({\text{A}}\) ' is :
323203 An organic compound ' \({\text{A}}\) ' containing \({\text{C, H}}\) and \({\text{O}}\) has a pleasant odour with boiling point \(78^{\circ} \mathrm{C}\). On boiling 'A' with conc \(\mathrm{H}_{2} \mathrm{SO}_{4}\), a colourless gas is released. The organic liquid ' \({\text{A}}\) ' is :
323203 An organic compound ' \({\text{A}}\) ' containing \({\text{C, H}}\) and \({\text{O}}\) has a pleasant odour with boiling point \(78^{\circ} \mathrm{C}\). On boiling 'A' with conc \(\mathrm{H}_{2} \mathrm{SO}_{4}\), a colourless gas is released. The organic liquid ' \({\text{A}}\) ' is :
323203 An organic compound ' \({\text{A}}\) ' containing \({\text{C, H}}\) and \({\text{O}}\) has a pleasant odour with boiling point \(78^{\circ} \mathrm{C}\). On boiling 'A' with conc \(\mathrm{H}_{2} \mathrm{SO}_{4}\), a colourless gas is released. The organic liquid ' \({\text{A}}\) ' is :