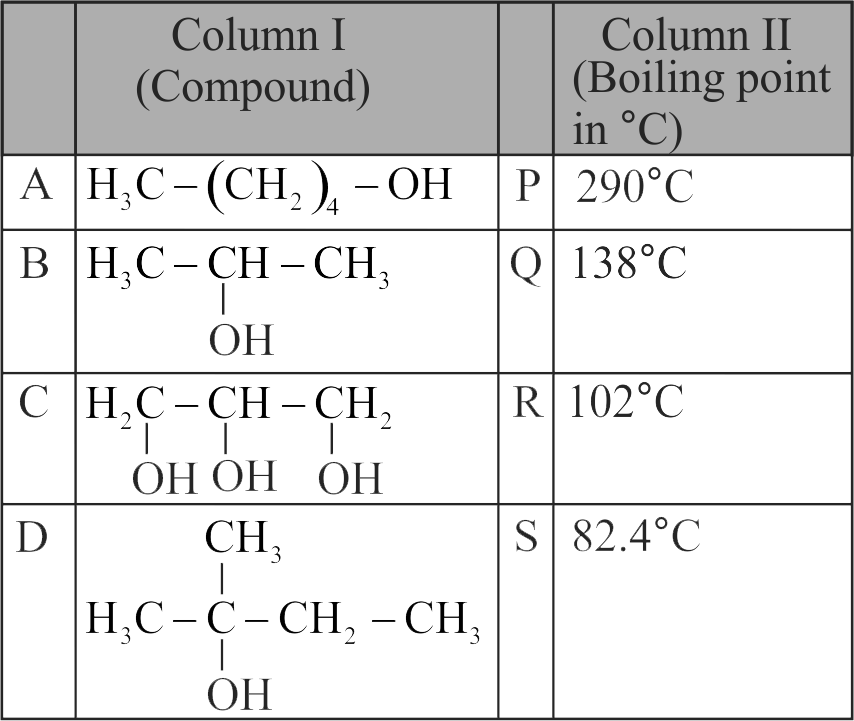
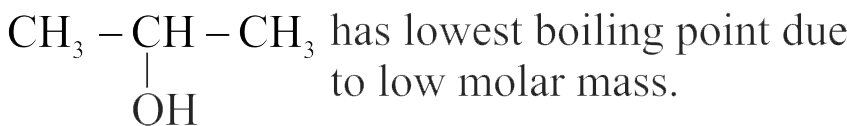323068
For the compounds,
(I) \({\mathrm{\mathrm{H}_{3} \mathrm{C}-\mathrm{CH}_{2}-\mathrm{O}-\mathrm{CH}_{2}-\mathrm{CH}_{2}-\mathrm{CH}_{3}}}\)
(II) \({\mathrm{\mathrm{H}_{3} \mathrm{C}-\mathrm{CH}_{2}-\mathrm{CH}_{2}-\mathrm{CH}_{2}-\mathrm{CH}_{3}}}\)
(III)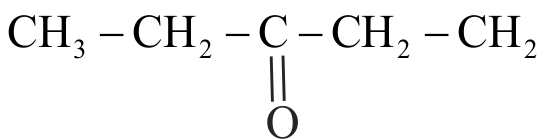
(IV)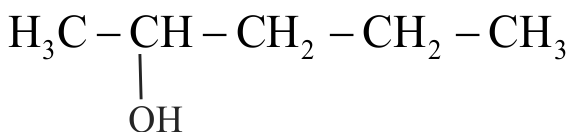
The increasing order of boiling point is Choose the correct answer from the options given below.
323068
For the compounds,
(I) \({\mathrm{\mathrm{H}_{3} \mathrm{C}-\mathrm{CH}_{2}-\mathrm{O}-\mathrm{CH}_{2}-\mathrm{CH}_{2}-\mathrm{CH}_{3}}}\)
(II) \({\mathrm{\mathrm{H}_{3} \mathrm{C}-\mathrm{CH}_{2}-\mathrm{CH}_{2}-\mathrm{CH}_{2}-\mathrm{CH}_{3}}}\)
(III)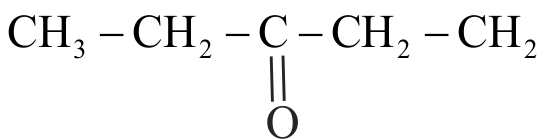
(IV)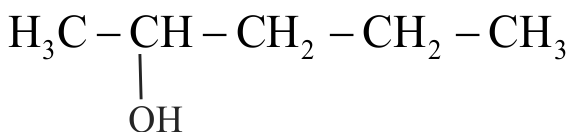
The increasing order of boiling point is Choose the correct answer from the options given below.
323068
For the compounds,
(I) \({\mathrm{\mathrm{H}_{3} \mathrm{C}-\mathrm{CH}_{2}-\mathrm{O}-\mathrm{CH}_{2}-\mathrm{CH}_{2}-\mathrm{CH}_{3}}}\)
(II) \({\mathrm{\mathrm{H}_{3} \mathrm{C}-\mathrm{CH}_{2}-\mathrm{CH}_{2}-\mathrm{CH}_{2}-\mathrm{CH}_{3}}}\)
(III)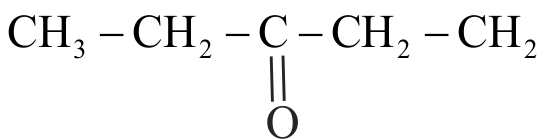
(IV)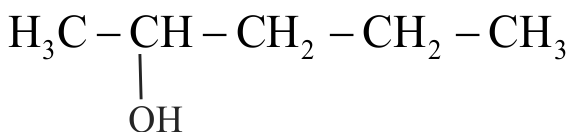
The increasing order of boiling point is Choose the correct answer from the options given below.
323068
For the compounds,
(I) \({\mathrm{\mathrm{H}_{3} \mathrm{C}-\mathrm{CH}_{2}-\mathrm{O}-\mathrm{CH}_{2}-\mathrm{CH}_{2}-\mathrm{CH}_{3}}}\)
(II) \({\mathrm{\mathrm{H}_{3} \mathrm{C}-\mathrm{CH}_{2}-\mathrm{CH}_{2}-\mathrm{CH}_{2}-\mathrm{CH}_{3}}}\)
(III)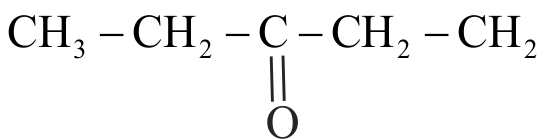
(IV)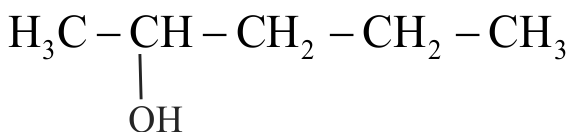
The increasing order of boiling point is Choose the correct answer from the options given below.