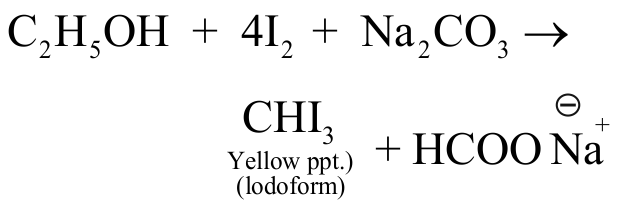322998 A compound corresponding to molecular formula \(\mathrm{C}_{4} \mathrm{H}_{10} \mathrm{O}\) does not give reaction with cold aqueous \(\mathrm{KMnO}_{4}\) but reacts with \(\mathrm{PCl}_{5}\) evolving HCl . The most likely structural formula of the compound is
322999 \(\mathrm{I}_{2}\) produced when ozone reacts with moist \(\mathrm{KI}\) is used to convert \(\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}\) to \(\mathrm{CI}_{3} \mathrm{CHO}\). Number of moles of ozone required to convert 1 mole of \(\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}\) into \(\mathrm{CI}_{3} \mathrm{CHO}\) is
322998 A compound corresponding to molecular formula \(\mathrm{C}_{4} \mathrm{H}_{10} \mathrm{O}\) does not give reaction with cold aqueous \(\mathrm{KMnO}_{4}\) but reacts with \(\mathrm{PCl}_{5}\) evolving HCl . The most likely structural formula of the compound is
322999 \(\mathrm{I}_{2}\) produced when ozone reacts with moist \(\mathrm{KI}\) is used to convert \(\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}\) to \(\mathrm{CI}_{3} \mathrm{CHO}\). Number of moles of ozone required to convert 1 mole of \(\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}\) into \(\mathrm{CI}_{3} \mathrm{CHO}\) is
322998 A compound corresponding to molecular formula \(\mathrm{C}_{4} \mathrm{H}_{10} \mathrm{O}\) does not give reaction with cold aqueous \(\mathrm{KMnO}_{4}\) but reacts with \(\mathrm{PCl}_{5}\) evolving HCl . The most likely structural formula of the compound is
322999 \(\mathrm{I}_{2}\) produced when ozone reacts with moist \(\mathrm{KI}\) is used to convert \(\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}\) to \(\mathrm{CI}_{3} \mathrm{CHO}\). Number of moles of ozone required to convert 1 mole of \(\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}\) into \(\mathrm{CI}_{3} \mathrm{CHO}\) is
322998 A compound corresponding to molecular formula \(\mathrm{C}_{4} \mathrm{H}_{10} \mathrm{O}\) does not give reaction with cold aqueous \(\mathrm{KMnO}_{4}\) but reacts with \(\mathrm{PCl}_{5}\) evolving HCl . The most likely structural formula of the compound is
322999 \(\mathrm{I}_{2}\) produced when ozone reacts with moist \(\mathrm{KI}\) is used to convert \(\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}\) to \(\mathrm{CI}_{3} \mathrm{CHO}\). Number of moles of ozone required to convert 1 mole of \(\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}\) into \(\mathrm{CI}_{3} \mathrm{CHO}\) is