322991
Given below are two statements :
Statement I :
In Lucas test, primary, secondary and tertiary alcohols are distinguished on the basis of their reactivity with conc.
Statement II:
Primary alcohols are most reactive and immediately produce turbidity at room temperature on reaction with Lucas Reagent.
In the light of the above statements, choose the most appropriate answer from the options given below:
322991
Given below are two statements :
Statement I :
In Lucas test, primary, secondary and tertiary alcohols are distinguished on the basis of their reactivity with conc.
Statement II:
Primary alcohols are most reactive and immediately produce turbidity at room temperature on reaction with Lucas Reagent.
In the light of the above statements, choose the most appropriate answer from the options given below:
322991
Given below are two statements :
Statement I :
In Lucas test, primary, secondary and tertiary alcohols are distinguished on the basis of their reactivity with conc.
Statement II:
Primary alcohols are most reactive and immediately produce turbidity at room temperature on reaction with Lucas Reagent.
In the light of the above statements, choose the most appropriate answer from the options given below:
322991
Given below are two statements :
Statement I :
In Lucas test, primary, secondary and tertiary alcohols are distinguished on the basis of their reactivity with conc.
Statement II:
Primary alcohols are most reactive and immediately produce turbidity at room temperature on reaction with Lucas Reagent.
In the light of the above statements, choose the most appropriate answer from the options given below:

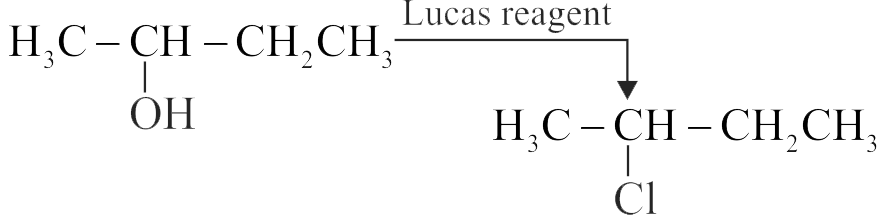
.png)
.png)