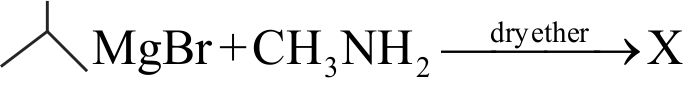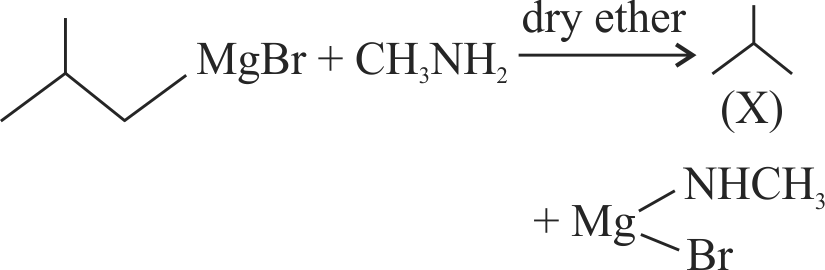322721
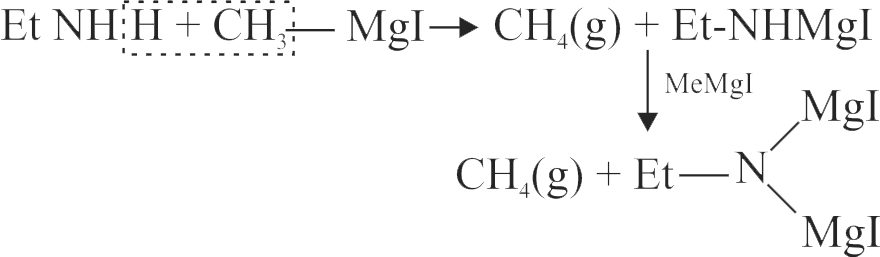
\({{\text{EtN}}{{\text{H}}_{\text{2}}}{\text{ + MeMgI}}\xrightarrow[{{\text{ temp}}{\text{. with pyridine }}}]{{{\text{Heated}}{\kern 1pt} {\kern 1pt} {\text{at}}{\kern 1pt} {\text{high}}}}{\text{Gas(A)}}}\)
The volume of gas (A) obtained at STP when
0.45 g of \({{\rm{EtN}}{{\rm{H}}_{\rm{2}}}}\) reacts with \({\text{ MeMgI}}\) is ____ .
322721
\({{\text{EtN}}{{\text{H}}_{\text{2}}}{\text{ + MeMgI}}\xrightarrow[{{\text{ temp}}{\text{. with pyridine }}}]{{{\text{Heated}}{\kern 1pt} {\kern 1pt} {\text{at}}{\kern 1pt} {\text{high}}}}{\text{Gas(A)}}}\)
The volume of gas (A) obtained at STP when
0.45 g of \({{\rm{EtN}}{{\rm{H}}_{\rm{2}}}}\) reacts with \({\text{ MeMgI}}\) is ____ .
322721
\({{\text{EtN}}{{\text{H}}_{\text{2}}}{\text{ + MeMgI}}\xrightarrow[{{\text{ temp}}{\text{. with pyridine }}}]{{{\text{Heated}}{\kern 1pt} {\kern 1pt} {\text{at}}{\kern 1pt} {\text{high}}}}{\text{Gas(A)}}}\)
The volume of gas (A) obtained at STP when
0.45 g of \({{\rm{EtN}}{{\rm{H}}_{\rm{2}}}}\) reacts with \({\text{ MeMgI}}\) is ____ .
322721
\({{\text{EtN}}{{\text{H}}_{\text{2}}}{\text{ + MeMgI}}\xrightarrow[{{\text{ temp}}{\text{. with pyridine }}}]{{{\text{Heated}}{\kern 1pt} {\kern 1pt} {\text{at}}{\kern 1pt} {\text{high}}}}{\text{Gas(A)}}}\)
The volume of gas (A) obtained at STP when
0.45 g of \({{\rm{EtN}}{{\rm{H}}_{\rm{2}}}}\) reacts with \({\text{ MeMgI}}\) is ____ .
322721
\({{\text{EtN}}{{\text{H}}_{\text{2}}}{\text{ + MeMgI}}\xrightarrow[{{\text{ temp}}{\text{. with pyridine }}}]{{{\text{Heated}}{\kern 1pt} {\kern 1pt} {\text{at}}{\kern 1pt} {\text{high}}}}{\text{Gas(A)}}}\)
The volume of gas (A) obtained at STP when
0.45 g of \({{\rm{EtN}}{{\rm{H}}_{\rm{2}}}}\) reacts with \({\text{ MeMgI}}\) is ____ .