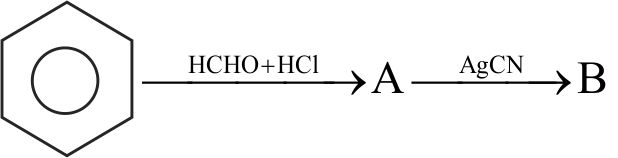
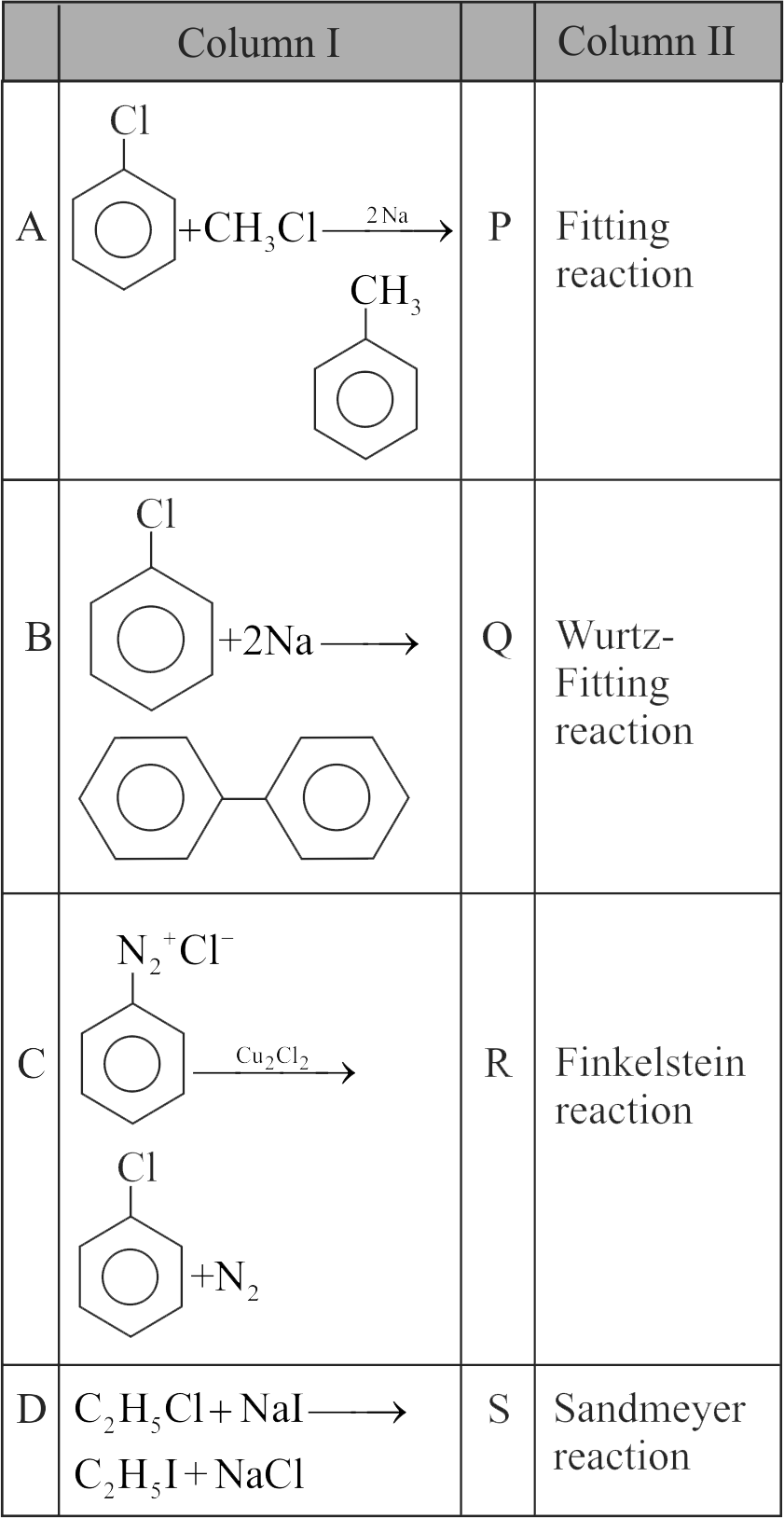
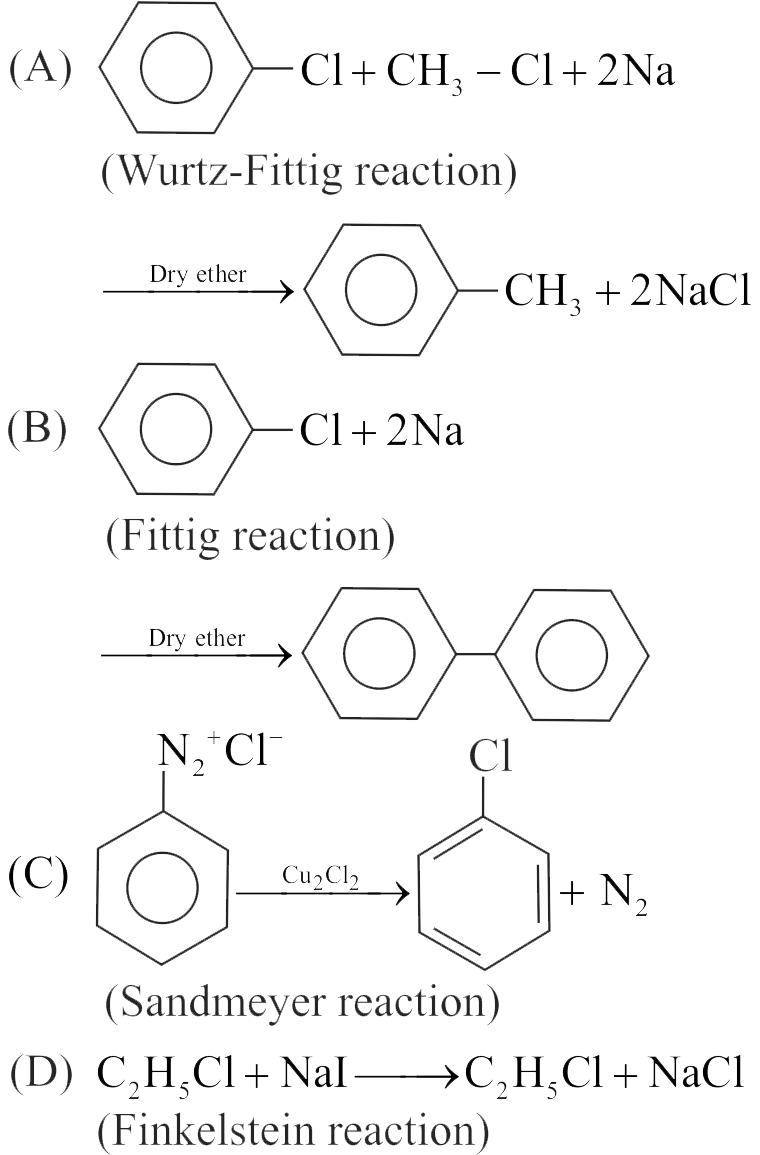
322560
Read the following statements and choose the correct answer.
(i) Alkyl iodide gives yellow ppt. with aq. \({\mathrm{\mathrm{AgNO}_{3}}}\) while aryl iodide does not.
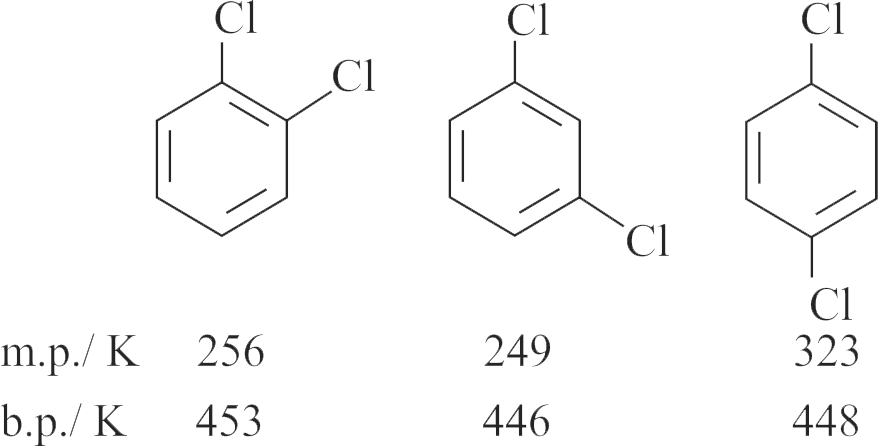
(ii) Among isomeric dihalobenzenes, the paraisomer have higher melting point than their ortho and meta isomers.
(iii) The isomeric dihalobenzenes have large difference in their boiling and melting points.
(iv) The isomeric dihalobenzenes have nearly same boiling point.
322560
Read the following statements and choose the correct answer.
(i) Alkyl iodide gives yellow ppt. with aq. \({\mathrm{\mathrm{AgNO}_{3}}}\) while aryl iodide does not.
(ii) Among isomeric dihalobenzenes, the paraisomer have higher melting point than their ortho and meta isomers.
(iii) The isomeric dihalobenzenes have large difference in their boiling and melting points.
(iv) The isomeric dihalobenzenes have nearly same boiling point.
322560
Read the following statements and choose the correct answer.
(i) Alkyl iodide gives yellow ppt. with aq. \({\mathrm{\mathrm{AgNO}_{3}}}\) while aryl iodide does not.
(ii) Among isomeric dihalobenzenes, the paraisomer have higher melting point than their ortho and meta isomers.
(iii) The isomeric dihalobenzenes have large difference in their boiling and melting points.
(iv) The isomeric dihalobenzenes have nearly same boiling point.
322560
Read the following statements and choose the correct answer.
(i) Alkyl iodide gives yellow ppt. with aq. \({\mathrm{\mathrm{AgNO}_{3}}}\) while aryl iodide does not.
(ii) Among isomeric dihalobenzenes, the paraisomer have higher melting point than their ortho and meta isomers.
(iii) The isomeric dihalobenzenes have large difference in their boiling and melting points.
(iv) The isomeric dihalobenzenes have nearly same boiling point.
322560
Read the following statements and choose the correct answer.
(i) Alkyl iodide gives yellow ppt. with aq. \({\mathrm{\mathrm{AgNO}_{3}}}\) while aryl iodide does not.
(ii) Among isomeric dihalobenzenes, the paraisomer have higher melting point than their ortho and meta isomers.
(iii) The isomeric dihalobenzenes have large difference in their boiling and melting points.
(iv) The isomeric dihalobenzenes have nearly same boiling point.