322552
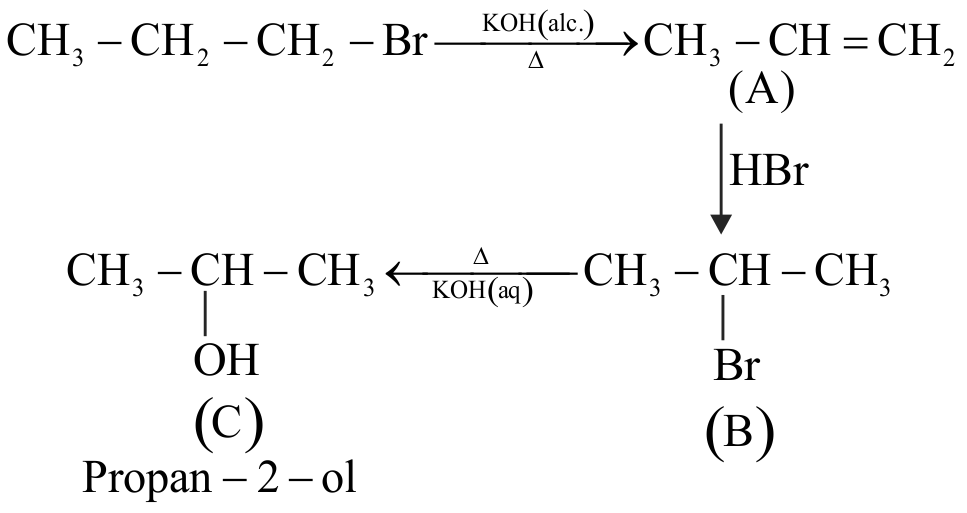
The product (C) in the below mentioned reaction is
\({\text{C}}{{\text{H}}_{\text{3}}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{Br}}\xrightarrow[\Delta ]{{{\text{KO}}{{\text{H}}_{{\text{(alc)}}}}}}{\text{A}}\)
\(\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\xrightarrow[\Delta ]{{{\text{HBr}}}}{\text{B}}\xrightarrow[{{\text{KO}}{{\text{H}}_{{\text{(aq)}}}}}]{\Delta }{\text{C}}\)
322553
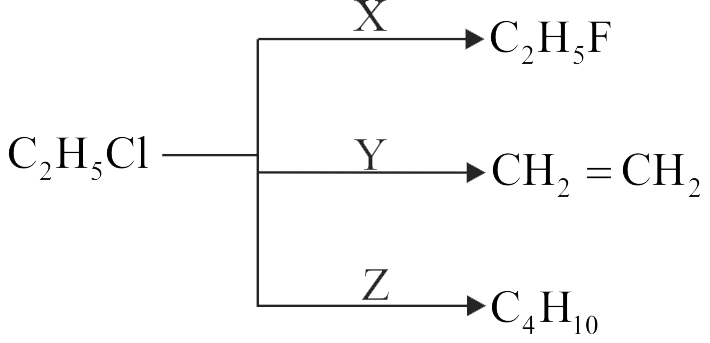
Identify \({\rm{Z}}\) in the following series:
\({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{I}}\xrightarrow{{{\text{AlC}} \cdot {\text{KOH}}}}{\text{X}}\xrightarrow{{{\text{B}}{{\text{r}}_{\text{2}}}}}{\text{Y}}\xrightarrow{{{\text{KCN}}}}{\text{Z}}\)
322554 A saturated alkyl halide \({\mathrm{\left(\mathrm{C}_{3} \mathrm{H}_{7} \mathrm{X}\right)}}\) heated with dry silver oxide \({\mathrm{\left(\mathrm{Ag}_{2} \mathrm{O}\right)}}\) forms 1-propoxypropane. The number of moles of alkyl halide consumed per mole of 1-propoxypropane formed is ____ .
322552
The product (C) in the below mentioned reaction is
\({\text{C}}{{\text{H}}_{\text{3}}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{Br}}\xrightarrow[\Delta ]{{{\text{KO}}{{\text{H}}_{{\text{(alc)}}}}}}{\text{A}}\)
\(\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\xrightarrow[\Delta ]{{{\text{HBr}}}}{\text{B}}\xrightarrow[{{\text{KO}}{{\text{H}}_{{\text{(aq)}}}}}]{\Delta }{\text{C}}\)
322553
Identify \({\rm{Z}}\) in the following series:
\({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{I}}\xrightarrow{{{\text{AlC}} \cdot {\text{KOH}}}}{\text{X}}\xrightarrow{{{\text{B}}{{\text{r}}_{\text{2}}}}}{\text{Y}}\xrightarrow{{{\text{KCN}}}}{\text{Z}}\)
322554 A saturated alkyl halide \({\mathrm{\left(\mathrm{C}_{3} \mathrm{H}_{7} \mathrm{X}\right)}}\) heated with dry silver oxide \({\mathrm{\left(\mathrm{Ag}_{2} \mathrm{O}\right)}}\) forms 1-propoxypropane. The number of moles of alkyl halide consumed per mole of 1-propoxypropane formed is ____ .
322552
The product (C) in the below mentioned reaction is
\({\text{C}}{{\text{H}}_{\text{3}}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{Br}}\xrightarrow[\Delta ]{{{\text{KO}}{{\text{H}}_{{\text{(alc)}}}}}}{\text{A}}\)
\(\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\xrightarrow[\Delta ]{{{\text{HBr}}}}{\text{B}}\xrightarrow[{{\text{KO}}{{\text{H}}_{{\text{(aq)}}}}}]{\Delta }{\text{C}}\)
322553
Identify \({\rm{Z}}\) in the following series:
\({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{I}}\xrightarrow{{{\text{AlC}} \cdot {\text{KOH}}}}{\text{X}}\xrightarrow{{{\text{B}}{{\text{r}}_{\text{2}}}}}{\text{Y}}\xrightarrow{{{\text{KCN}}}}{\text{Z}}\)
322554 A saturated alkyl halide \({\mathrm{\left(\mathrm{C}_{3} \mathrm{H}_{7} \mathrm{X}\right)}}\) heated with dry silver oxide \({\mathrm{\left(\mathrm{Ag}_{2} \mathrm{O}\right)}}\) forms 1-propoxypropane. The number of moles of alkyl halide consumed per mole of 1-propoxypropane formed is ____ .
322552
The product (C) in the below mentioned reaction is
\({\text{C}}{{\text{H}}_{\text{3}}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{Br}}\xrightarrow[\Delta ]{{{\text{KO}}{{\text{H}}_{{\text{(alc)}}}}}}{\text{A}}\)
\(\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\xrightarrow[\Delta ]{{{\text{HBr}}}}{\text{B}}\xrightarrow[{{\text{KO}}{{\text{H}}_{{\text{(aq)}}}}}]{\Delta }{\text{C}}\)
322553
Identify \({\rm{Z}}\) in the following series:
\({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{I}}\xrightarrow{{{\text{AlC}} \cdot {\text{KOH}}}}{\text{X}}\xrightarrow{{{\text{B}}{{\text{r}}_{\text{2}}}}}{\text{Y}}\xrightarrow{{{\text{KCN}}}}{\text{Z}}\)
322554 A saturated alkyl halide \({\mathrm{\left(\mathrm{C}_{3} \mathrm{H}_{7} \mathrm{X}\right)}}\) heated with dry silver oxide \({\mathrm{\left(\mathrm{Ag}_{2} \mathrm{O}\right)}}\) forms 1-propoxypropane. The number of moles of alkyl halide consumed per mole of 1-propoxypropane formed is ____ .