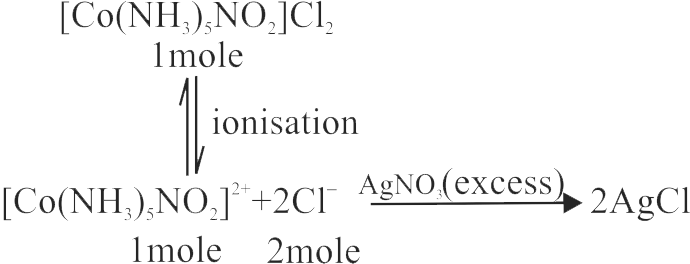
322067 A coordination compound in its molecular formula contains five ammonia molecules, one nitro group, two chloride ions and one cobalt ion. One mole of the complex salt produces three moles of ions in its aqueous solution. With excess of \(\mathrm{AgNO}_{3}\) solution, one mole of it gives two moles of AgCl ppt. Formula of the complex is
322067 A coordination compound in its molecular formula contains five ammonia molecules, one nitro group, two chloride ions and one cobalt ion. One mole of the complex salt produces three moles of ions in its aqueous solution. With excess of \(\mathrm{AgNO}_{3}\) solution, one mole of it gives two moles of AgCl ppt. Formula of the complex is
322067 A coordination compound in its molecular formula contains five ammonia molecules, one nitro group, two chloride ions and one cobalt ion. One mole of the complex salt produces three moles of ions in its aqueous solution. With excess of \(\mathrm{AgNO}_{3}\) solution, one mole of it gives two moles of AgCl ppt. Formula of the complex is
322067 A coordination compound in its molecular formula contains five ammonia molecules, one nitro group, two chloride ions and one cobalt ion. One mole of the complex salt produces three moles of ions in its aqueous solution. With excess of \(\mathrm{AgNO}_{3}\) solution, one mole of it gives two moles of AgCl ppt. Formula of the complex is
322067 A coordination compound in its molecular formula contains five ammonia molecules, one nitro group, two chloride ions and one cobalt ion. One mole of the complex salt produces three moles of ions in its aqueous solution. With excess of \(\mathrm{AgNO}_{3}\) solution, one mole of it gives two moles of AgCl ppt. Formula of the complex is