321958
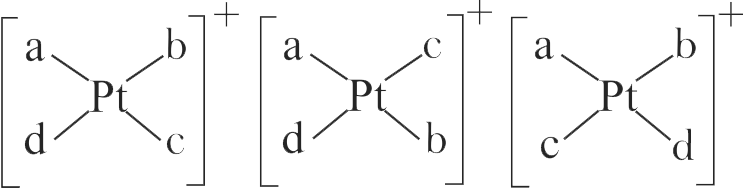
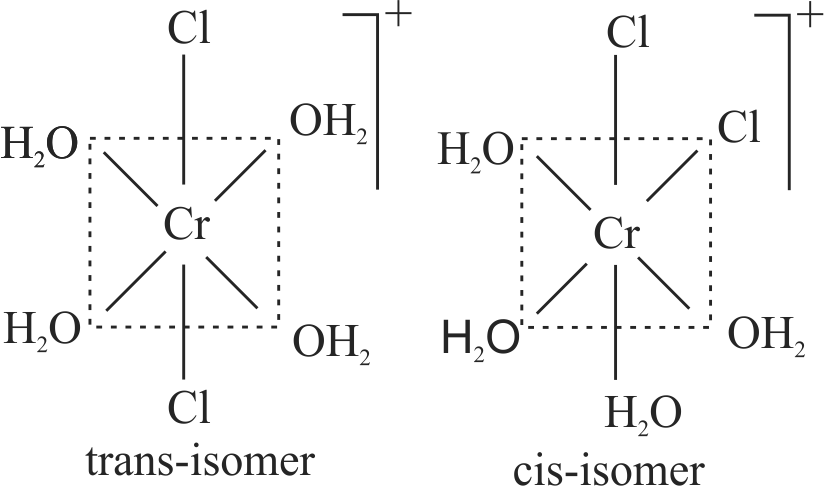
The correct statement on the isomerism associated with the following complex ions
(A) \(\left[\mathrm{Ni}\left(\mathrm{H}_{2} \mathrm{O}\right)_{5} \mathrm{NH}_{3}\right]^{2+}\)
(B) \(\left[\mathrm{Ni}\left(\mathrm{H}_{2} \mathrm{O}\right)_{4}\left(\mathrm{NH}_{3}\right)_{2}\right]^{2+}\) and
(C) \(\left[\mathrm{Ni}\left(\mathrm{H}_{2} \mathrm{O}\right)_{3}\left(\mathrm{NH}_{3}\right)_{3}\right]^{2+}\) is
321958
The correct statement on the isomerism associated with the following complex ions
(A) \(\left[\mathrm{Ni}\left(\mathrm{H}_{2} \mathrm{O}\right)_{5} \mathrm{NH}_{3}\right]^{2+}\)
(B) \(\left[\mathrm{Ni}\left(\mathrm{H}_{2} \mathrm{O}\right)_{4}\left(\mathrm{NH}_{3}\right)_{2}\right]^{2+}\) and
(C) \(\left[\mathrm{Ni}\left(\mathrm{H}_{2} \mathrm{O}\right)_{3}\left(\mathrm{NH}_{3}\right)_{3}\right]^{2+}\) is
321958
The correct statement on the isomerism associated with the following complex ions
(A) \(\left[\mathrm{Ni}\left(\mathrm{H}_{2} \mathrm{O}\right)_{5} \mathrm{NH}_{3}\right]^{2+}\)
(B) \(\left[\mathrm{Ni}\left(\mathrm{H}_{2} \mathrm{O}\right)_{4}\left(\mathrm{NH}_{3}\right)_{2}\right]^{2+}\) and
(C) \(\left[\mathrm{Ni}\left(\mathrm{H}_{2} \mathrm{O}\right)_{3}\left(\mathrm{NH}_{3}\right)_{3}\right]^{2+}\) is
321958
The correct statement on the isomerism associated with the following complex ions
(A) \(\left[\mathrm{Ni}\left(\mathrm{H}_{2} \mathrm{O}\right)_{5} \mathrm{NH}_{3}\right]^{2+}\)
(B) \(\left[\mathrm{Ni}\left(\mathrm{H}_{2} \mathrm{O}\right)_{4}\left(\mathrm{NH}_{3}\right)_{2}\right]^{2+}\) and
(C) \(\left[\mathrm{Ni}\left(\mathrm{H}_{2} \mathrm{O}\right)_{3}\left(\mathrm{NH}_{3}\right)_{3}\right]^{2+}\) is