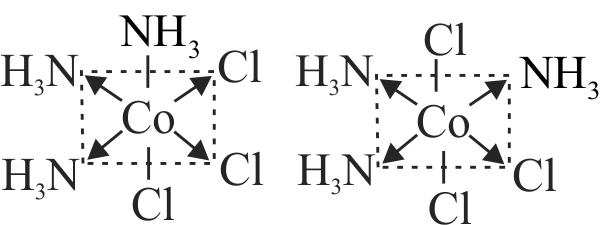
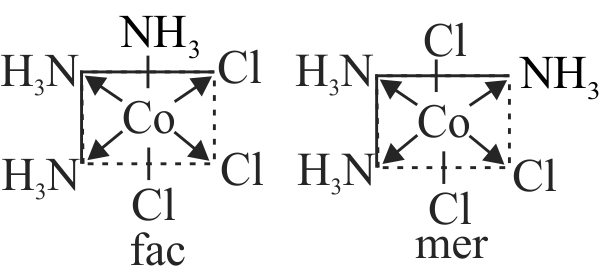
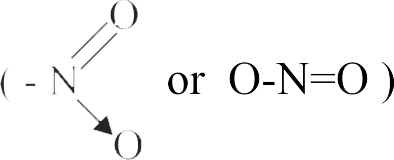
322007 What kind of isomerism exists between \(\left[\mathrm{Cr}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right] \mathrm{Cl}_{3}\) (violet) and \(\left[\mathrm{Cr}\left(\mathrm{H}_{2} \mathrm{O}\right)_{5} \mathrm{Cl}\right] \mathrm{Cl}_{2}\). \(\mathrm{H}_{2} \mathrm{O}\) (greyish-green)?
322007 What kind of isomerism exists between \(\left[\mathrm{Cr}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right] \mathrm{Cl}_{3}\) (violet) and \(\left[\mathrm{Cr}\left(\mathrm{H}_{2} \mathrm{O}\right)_{5} \mathrm{Cl}\right] \mathrm{Cl}_{2}\). \(\mathrm{H}_{2} \mathrm{O}\) (greyish-green)?
322007 What kind of isomerism exists between \(\left[\mathrm{Cr}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right] \mathrm{Cl}_{3}\) (violet) and \(\left[\mathrm{Cr}\left(\mathrm{H}_{2} \mathrm{O}\right)_{5} \mathrm{Cl}\right] \mathrm{Cl}_{2}\). \(\mathrm{H}_{2} \mathrm{O}\) (greyish-green)?
322007 What kind of isomerism exists between \(\left[\mathrm{Cr}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right] \mathrm{Cl}_{3}\) (violet) and \(\left[\mathrm{Cr}\left(\mathrm{H}_{2} \mathrm{O}\right)_{5} \mathrm{Cl}\right] \mathrm{Cl}_{2}\). \(\mathrm{H}_{2} \mathrm{O}\) (greyish-green)?