321976
Which of the following compound show optical isomerism? \((\mathrm{en}=\) ethylenediamine \()\)
(I) \(\mathrm{cis}\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{4} \mathrm{Cl}_{2}\right]\)
(II) trans \(-\left[\mathrm{Co}(\mathrm{en})_{2} \mathrm{Cl}_{2}\right]\)
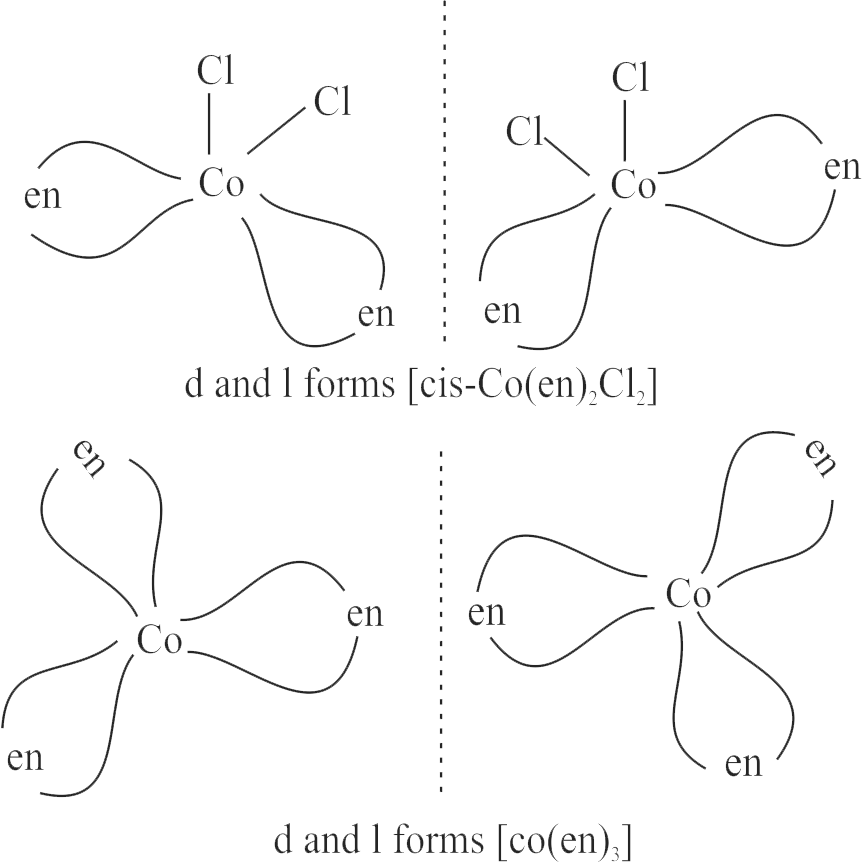
(III) \(\operatorname{cis}\left[\mathrm{Co}(\mathrm{en})_{2} \mathrm{Cl}_{2}\right]\)
(IV) \(\left[\operatorname{Co}(\mathrm{en})_{3}\right]\)
Select the correct answer using codes given below.
321976
Which of the following compound show optical isomerism? \((\mathrm{en}=\) ethylenediamine \()\)
(I) \(\mathrm{cis}\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{4} \mathrm{Cl}_{2}\right]\)
(II) trans \(-\left[\mathrm{Co}(\mathrm{en})_{2} \mathrm{Cl}_{2}\right]\)
(III) \(\operatorname{cis}\left[\mathrm{Co}(\mathrm{en})_{2} \mathrm{Cl}_{2}\right]\)
(IV) \(\left[\operatorname{Co}(\mathrm{en})_{3}\right]\)
Select the correct answer using codes given below.
321976
Which of the following compound show optical isomerism? \((\mathrm{en}=\) ethylenediamine \()\)
(I) \(\mathrm{cis}\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{4} \mathrm{Cl}_{2}\right]\)
(II) trans \(-\left[\mathrm{Co}(\mathrm{en})_{2} \mathrm{Cl}_{2}\right]\)
(III) \(\operatorname{cis}\left[\mathrm{Co}(\mathrm{en})_{2} \mathrm{Cl}_{2}\right]\)
(IV) \(\left[\operatorname{Co}(\mathrm{en})_{3}\right]\)
Select the correct answer using codes given below.
321976
Which of the following compound show optical isomerism? \((\mathrm{en}=\) ethylenediamine \()\)
(I) \(\mathrm{cis}\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{4} \mathrm{Cl}_{2}\right]\)
(II) trans \(-\left[\mathrm{Co}(\mathrm{en})_{2} \mathrm{Cl}_{2}\right]\)
(III) \(\operatorname{cis}\left[\mathrm{Co}(\mathrm{en})_{2} \mathrm{Cl}_{2}\right]\)
(IV) \(\left[\operatorname{Co}(\mathrm{en})_{3}\right]\)
Select the correct answer using codes given below.