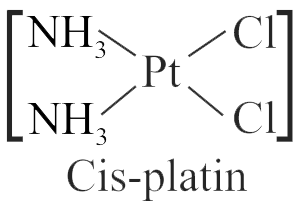
321893
The mismatched combinations are
A. Chlorophyll - Co
B. Water hardness - EDTA
C. Photography \(-\left[\operatorname{Ag}(\mathrm{CN})_{2}\right]\)
D. Wilkinson's catalyst - \(\left[\left(\mathrm{Ph}_{3} \mathrm{P}\right)_{3} \mathrm{RhCl}\right]\)
E. Chelating ligand -D-Penicillamine
Choose the correct answer from the options given below :
321894 \({{\text{C}}_{{\text{63}}}}{{\text{H}}_{{\text{88}}}}{\text{CO}}{{\text{N}}_{{\text{14}}}}{{\text{O}}_{{\text{14}}}}{\text{P}}\) is the formulae of the Cyanocobalamine ,(vitamin \({{\text{B}}_{{\text{12}}}}\)) it contain \(\mathrm{CN}^{-}\) is very poisonous ,than why this compound does not prove to be fatal for us ?(it inhibit the electron transport chain?
321893
The mismatched combinations are
A. Chlorophyll - Co
B. Water hardness - EDTA
C. Photography \(-\left[\operatorname{Ag}(\mathrm{CN})_{2}\right]\)
D. Wilkinson's catalyst - \(\left[\left(\mathrm{Ph}_{3} \mathrm{P}\right)_{3} \mathrm{RhCl}\right]\)
E. Chelating ligand -D-Penicillamine
Choose the correct answer from the options given below :
321894 \({{\text{C}}_{{\text{63}}}}{{\text{H}}_{{\text{88}}}}{\text{CO}}{{\text{N}}_{{\text{14}}}}{{\text{O}}_{{\text{14}}}}{\text{P}}\) is the formulae of the Cyanocobalamine ,(vitamin \({{\text{B}}_{{\text{12}}}}\)) it contain \(\mathrm{CN}^{-}\) is very poisonous ,than why this compound does not prove to be fatal for us ?(it inhibit the electron transport chain?
321893
The mismatched combinations are
A. Chlorophyll - Co
B. Water hardness - EDTA
C. Photography \(-\left[\operatorname{Ag}(\mathrm{CN})_{2}\right]\)
D. Wilkinson's catalyst - \(\left[\left(\mathrm{Ph}_{3} \mathrm{P}\right)_{3} \mathrm{RhCl}\right]\)
E. Chelating ligand -D-Penicillamine
Choose the correct answer from the options given below :
321894 \({{\text{C}}_{{\text{63}}}}{{\text{H}}_{{\text{88}}}}{\text{CO}}{{\text{N}}_{{\text{14}}}}{{\text{O}}_{{\text{14}}}}{\text{P}}\) is the formulae of the Cyanocobalamine ,(vitamin \({{\text{B}}_{{\text{12}}}}\)) it contain \(\mathrm{CN}^{-}\) is very poisonous ,than why this compound does not prove to be fatal for us ?(it inhibit the electron transport chain?
321893
The mismatched combinations are
A. Chlorophyll - Co
B. Water hardness - EDTA
C. Photography \(-\left[\operatorname{Ag}(\mathrm{CN})_{2}\right]\)
D. Wilkinson's catalyst - \(\left[\left(\mathrm{Ph}_{3} \mathrm{P}\right)_{3} \mathrm{RhCl}\right]\)
E. Chelating ligand -D-Penicillamine
Choose the correct answer from the options given below :
321894 \({{\text{C}}_{{\text{63}}}}{{\text{H}}_{{\text{88}}}}{\text{CO}}{{\text{N}}_{{\text{14}}}}{{\text{O}}_{{\text{14}}}}{\text{P}}\) is the formulae of the Cyanocobalamine ,(vitamin \({{\text{B}}_{{\text{12}}}}\)) it contain \(\mathrm{CN}^{-}\) is very poisonous ,than why this compound does not prove to be fatal for us ?(it inhibit the electron transport chain?
321893
The mismatched combinations are
A. Chlorophyll - Co
B. Water hardness - EDTA
C. Photography \(-\left[\operatorname{Ag}(\mathrm{CN})_{2}\right]\)
D. Wilkinson's catalyst - \(\left[\left(\mathrm{Ph}_{3} \mathrm{P}\right)_{3} \mathrm{RhCl}\right]\)
E. Chelating ligand -D-Penicillamine
Choose the correct answer from the options given below :
321894 \({{\text{C}}_{{\text{63}}}}{{\text{H}}_{{\text{88}}}}{\text{CO}}{{\text{N}}_{{\text{14}}}}{{\text{O}}_{{\text{14}}}}{\text{P}}\) is the formulae of the Cyanocobalamine ,(vitamin \({{\text{B}}_{{\text{12}}}}\)) it contain \(\mathrm{CN}^{-}\) is very poisonous ,than why this compound does not prove to be fatal for us ?(it inhibit the electron transport chain?