321818
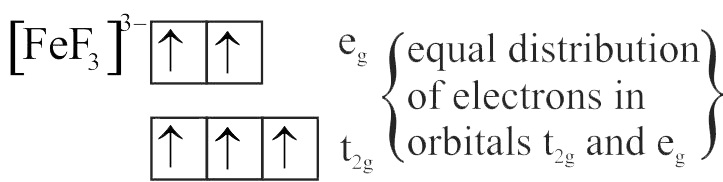
Number of complexes with even number of electrons in \({\mathrm{\mathrm{t}_{2 \mathrm{~g}}}}\) orbitals is
\[\begin{array}{l}
{\left[ {{\rm{Fe}}{{\left( {{{\rm{H}}_2}{\rm{O}}} \right)}_6}} \right]^{2 + }},{\left[ {{\rm{Co}}{{\left( {{{\rm{H}}_2}{\rm{O}}} \right)}_6}} \right]^{2 + }},{\left[ {{\rm{Co}}{{\left( {{{\rm{H}}_2}{\rm{O}}} \right)}_6}} \right]^{3 + }},\\
{\left[ {{\rm{Cu}}{{\left( {{{\rm{H}}_2}{\rm{O}}} \right)}_6}} \right]^{2 + }},{\left[ {{\rm{Cr}}{{\left( {{{\rm{H}}_2}{\rm{O}}} \right)}_6}} \right]^{2 + }}
\end{array}\]
321818
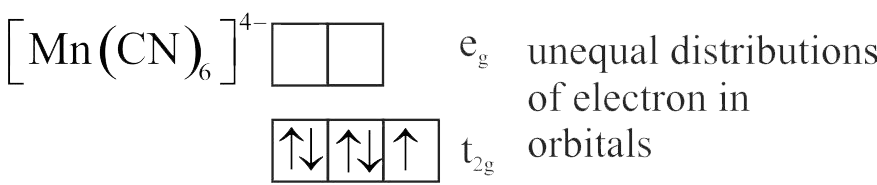
Number of complexes with even number of electrons in \({\mathrm{\mathrm{t}_{2 \mathrm{~g}}}}\) orbitals is
\[\begin{array}{l}
{\left[ {{\rm{Fe}}{{\left( {{{\rm{H}}_2}{\rm{O}}} \right)}_6}} \right]^{2 + }},{\left[ {{\rm{Co}}{{\left( {{{\rm{H}}_2}{\rm{O}}} \right)}_6}} \right]^{2 + }},{\left[ {{\rm{Co}}{{\left( {{{\rm{H}}_2}{\rm{O}}} \right)}_6}} \right]^{3 + }},\\
{\left[ {{\rm{Cu}}{{\left( {{{\rm{H}}_2}{\rm{O}}} \right)}_6}} \right]^{2 + }},{\left[ {{\rm{Cr}}{{\left( {{{\rm{H}}_2}{\rm{O}}} \right)}_6}} \right]^{2 + }}
\end{array}\]
321818
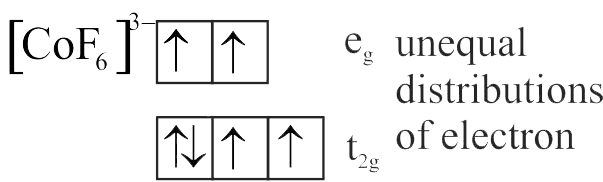
Number of complexes with even number of electrons in \({\mathrm{\mathrm{t}_{2 \mathrm{~g}}}}\) orbitals is
\[\begin{array}{l}
{\left[ {{\rm{Fe}}{{\left( {{{\rm{H}}_2}{\rm{O}}} \right)}_6}} \right]^{2 + }},{\left[ {{\rm{Co}}{{\left( {{{\rm{H}}_2}{\rm{O}}} \right)}_6}} \right]^{2 + }},{\left[ {{\rm{Co}}{{\left( {{{\rm{H}}_2}{\rm{O}}} \right)}_6}} \right]^{3 + }},\\
{\left[ {{\rm{Cu}}{{\left( {{{\rm{H}}_2}{\rm{O}}} \right)}_6}} \right]^{2 + }},{\left[ {{\rm{Cr}}{{\left( {{{\rm{H}}_2}{\rm{O}}} \right)}_6}} \right]^{2 + }}
\end{array}\]
321818
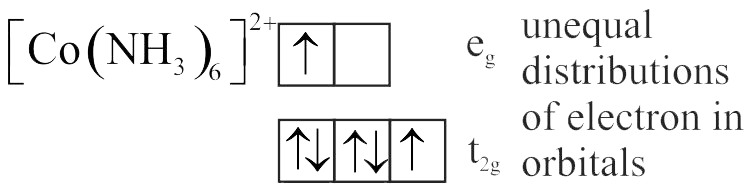
Number of complexes with even number of electrons in \({\mathrm{\mathrm{t}_{2 \mathrm{~g}}}}\) orbitals is
\[\begin{array}{l}
{\left[ {{\rm{Fe}}{{\left( {{{\rm{H}}_2}{\rm{O}}} \right)}_6}} \right]^{2 + }},{\left[ {{\rm{Co}}{{\left( {{{\rm{H}}_2}{\rm{O}}} \right)}_6}} \right]^{2 + }},{\left[ {{\rm{Co}}{{\left( {{{\rm{H}}_2}{\rm{O}}} \right)}_6}} \right]^{3 + }},\\
{\left[ {{\rm{Cu}}{{\left( {{{\rm{H}}_2}{\rm{O}}} \right)}_6}} \right]^{2 + }},{\left[ {{\rm{Cr}}{{\left( {{{\rm{H}}_2}{\rm{O}}} \right)}_6}} \right]^{2 + }}
\end{array}\]
321818
Number of complexes with even number of electrons in \({\mathrm{\mathrm{t}_{2 \mathrm{~g}}}}\) orbitals is
\[\begin{array}{l}
{\left[ {{\rm{Fe}}{{\left( {{{\rm{H}}_2}{\rm{O}}} \right)}_6}} \right]^{2 + }},{\left[ {{\rm{Co}}{{\left( {{{\rm{H}}_2}{\rm{O}}} \right)}_6}} \right]^{2 + }},{\left[ {{\rm{Co}}{{\left( {{{\rm{H}}_2}{\rm{O}}} \right)}_6}} \right]^{3 + }},\\
{\left[ {{\rm{Cu}}{{\left( {{{\rm{H}}_2}{\rm{O}}} \right)}_6}} \right]^{2 + }},{\left[ {{\rm{Cr}}{{\left( {{{\rm{H}}_2}{\rm{O}}} \right)}_6}} \right]^{2 + }}
\end{array}\]