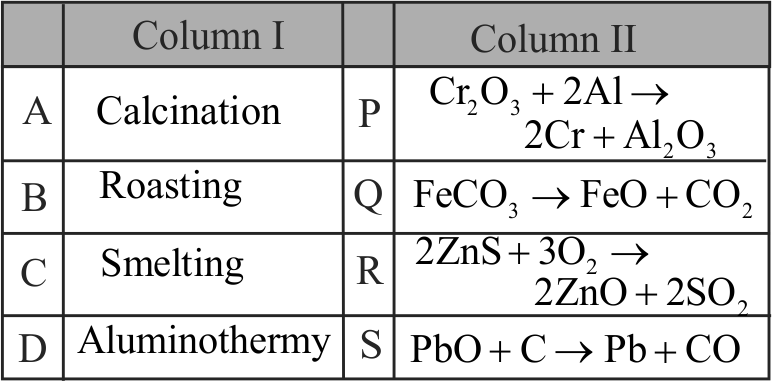
321065
Match items of Column I with the items of Column II and assign the correct code
Column I
Column II
A
Cyanide process
1
Ultra/ pure Ge
B
Froth floatation process
2
Dressing of ZnS
C
Electrolytic reduction
3
Extraction of Al
D
Zone refining
4
Extraction of Au
5
Purification of Ni
321066
Match the column I with column II to match the method of extraction and mark the appropriate choice.
Column I
Column II
A
\(\mathrm{\mathrm{Cu}}\)
P
\(\mathrm{\begin{array}{l}\text { Direct reduction of } \\\text { sulphide by heating }\end{array}}\)
B
\(\mathrm{\mathrm{Sn}}\)
Q
\(\mathrm{\begin{array}{l}\text { Electrolysis of fused } \\\text { chloride and fluoride }\end{array}}\)
C
\(\mathrm{\mathrm{Hg}}\)
R
\(\mathrm{\begin{array}{l}\text {Partial oxidation of } \\\text { sulphide ore }\end{array}}\)
D
\(\mathrm{\mathrm{Ca}}\)
P
\(\mathrm{\begin{array}{l}\text {Reduction of oxide} \\\text {with carbon }\end{array}}\)
321065
Match items of Column I with the items of Column II and assign the correct code
Column I
Column II
A
Cyanide process
1
Ultra/ pure Ge
B
Froth floatation process
2
Dressing of ZnS
C
Electrolytic reduction
3
Extraction of Al
D
Zone refining
4
Extraction of Au
5
Purification of Ni
321066
Match the column I with column II to match the method of extraction and mark the appropriate choice.
Column I
Column II
A
\(\mathrm{\mathrm{Cu}}\)
P
\(\mathrm{\begin{array}{l}\text { Direct reduction of } \\\text { sulphide by heating }\end{array}}\)
B
\(\mathrm{\mathrm{Sn}}\)
Q
\(\mathrm{\begin{array}{l}\text { Electrolysis of fused } \\\text { chloride and fluoride }\end{array}}\)
C
\(\mathrm{\mathrm{Hg}}\)
R
\(\mathrm{\begin{array}{l}\text {Partial oxidation of } \\\text { sulphide ore }\end{array}}\)
D
\(\mathrm{\mathrm{Ca}}\)
P
\(\mathrm{\begin{array}{l}\text {Reduction of oxide} \\\text {with carbon }\end{array}}\)
321065
Match items of Column I with the items of Column II and assign the correct code
Column I
Column II
A
Cyanide process
1
Ultra/ pure Ge
B
Froth floatation process
2
Dressing of ZnS
C
Electrolytic reduction
3
Extraction of Al
D
Zone refining
4
Extraction of Au
5
Purification of Ni
321066
Match the column I with column II to match the method of extraction and mark the appropriate choice.
Column I
Column II
A
\(\mathrm{\mathrm{Cu}}\)
P
\(\mathrm{\begin{array}{l}\text { Direct reduction of } \\\text { sulphide by heating }\end{array}}\)
B
\(\mathrm{\mathrm{Sn}}\)
Q
\(\mathrm{\begin{array}{l}\text { Electrolysis of fused } \\\text { chloride and fluoride }\end{array}}\)
C
\(\mathrm{\mathrm{Hg}}\)
R
\(\mathrm{\begin{array}{l}\text {Partial oxidation of } \\\text { sulphide ore }\end{array}}\)
D
\(\mathrm{\mathrm{Ca}}\)
P
\(\mathrm{\begin{array}{l}\text {Reduction of oxide} \\\text {with carbon }\end{array}}\)
321065
Match items of Column I with the items of Column II and assign the correct code
Column I
Column II
A
Cyanide process
1
Ultra/ pure Ge
B
Froth floatation process
2
Dressing of ZnS
C
Electrolytic reduction
3
Extraction of Al
D
Zone refining
4
Extraction of Au
5
Purification of Ni
321066
Match the column I with column II to match the method of extraction and mark the appropriate choice.
Column I
Column II
A
\(\mathrm{\mathrm{Cu}}\)
P
\(\mathrm{\begin{array}{l}\text { Direct reduction of } \\\text { sulphide by heating }\end{array}}\)
B
\(\mathrm{\mathrm{Sn}}\)
Q
\(\mathrm{\begin{array}{l}\text { Electrolysis of fused } \\\text { chloride and fluoride }\end{array}}\)
C
\(\mathrm{\mathrm{Hg}}\)
R
\(\mathrm{\begin{array}{l}\text {Partial oxidation of } \\\text { sulphide ore }\end{array}}\)
D
\(\mathrm{\mathrm{Ca}}\)
P
\(\mathrm{\begin{array}{l}\text {Reduction of oxide} \\\text {with carbon }\end{array}}\)
321065
Match items of Column I with the items of Column II and assign the correct code
Column I
Column II
A
Cyanide process
1
Ultra/ pure Ge
B
Froth floatation process
2
Dressing of ZnS
C
Electrolytic reduction
3
Extraction of Al
D
Zone refining
4
Extraction of Au
5
Purification of Ni
321066
Match the column I with column II to match the method of extraction and mark the appropriate choice.
Column I
Column II
A
\(\mathrm{\mathrm{Cu}}\)
P
\(\mathrm{\begin{array}{l}\text { Direct reduction of } \\\text { sulphide by heating }\end{array}}\)
B
\(\mathrm{\mathrm{Sn}}\)
Q
\(\mathrm{\begin{array}{l}\text { Electrolysis of fused } \\\text { chloride and fluoride }\end{array}}\)
C
\(\mathrm{\mathrm{Hg}}\)
R
\(\mathrm{\begin{array}{l}\text {Partial oxidation of } \\\text { sulphide ore }\end{array}}\)
D
\(\mathrm{\mathrm{Ca}}\)
P
\(\mathrm{\begin{array}{l}\text {Reduction of oxide} \\\text {with carbon }\end{array}}\)