320258
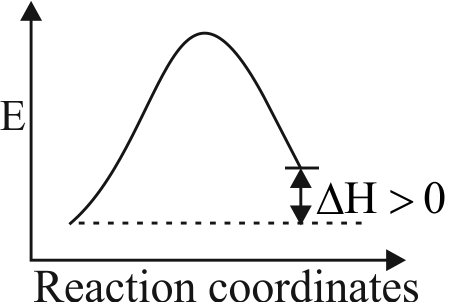
Assertion :
The overall rate of a reversible reaction may decrease with the increase in temperature.
Reason :
When the activation energy of forward reaction is less than that of backward reaction, then the increase in the rate of backward reaction is more than that of forward reaction on increasing the temperature.
320258
Assertion :
The overall rate of a reversible reaction may decrease with the increase in temperature.
Reason :
When the activation energy of forward reaction is less than that of backward reaction, then the increase in the rate of backward reaction is more than that of forward reaction on increasing the temperature.
320258
Assertion :
The overall rate of a reversible reaction may decrease with the increase in temperature.
Reason :
When the activation energy of forward reaction is less than that of backward reaction, then the increase in the rate of backward reaction is more than that of forward reaction on increasing the temperature.
320258
Assertion :
The overall rate of a reversible reaction may decrease with the increase in temperature.
Reason :
When the activation energy of forward reaction is less than that of backward reaction, then the increase in the rate of backward reaction is more than that of forward reaction on increasing the temperature.