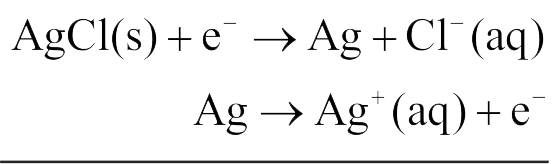330062
What will be the emf of the following concentration cell at \({\rm{25^\circ C}}\)?
\(\left. {{\rm{A}}{{\rm{g}}_{\left( {\rm{s}} \right)}}} \right \vert \left. {{\rm{AgN}}{{\rm{O}}_{\rm{3}}}{\rm{(0}}{\rm{.01M)}}} \right\vert \left. {{\rm{AgN}}{{\rm{O}}_{\rm{3}}}{\rm{(0}}{\rm{.05M)}}} \right \vert {\rm{A}}{{\rm{g}}_{{\rm{(s)}}}}\)
330064 Standard electrode potential for \({\rm{S}}{{\rm{n}}^{{\rm{4 + }}}}{\rm{/S}}{{\rm{n}}^{{\rm{2 + }}}}\) couple is \({\rm{ + 0}}{\rm{.15}}\,\,{\rm{V}}\) and that for the \({\rm{C}}{{\rm{r}}^{{\rm{3 + }}}}{\rm{/Cr}}\) couple is \({\rm{ - 0}}{\rm{.74}}\,\,{\rm{V}}\). These two couple in their standard state are connected to make a cell. The cell potential will be
330062
What will be the emf of the following concentration cell at \({\rm{25^\circ C}}\)?
\(\left. {{\rm{A}}{{\rm{g}}_{\left( {\rm{s}} \right)}}} \right \vert \left. {{\rm{AgN}}{{\rm{O}}_{\rm{3}}}{\rm{(0}}{\rm{.01M)}}} \right\vert \left. {{\rm{AgN}}{{\rm{O}}_{\rm{3}}}{\rm{(0}}{\rm{.05M)}}} \right \vert {\rm{A}}{{\rm{g}}_{{\rm{(s)}}}}\)
330064 Standard electrode potential for \({\rm{S}}{{\rm{n}}^{{\rm{4 + }}}}{\rm{/S}}{{\rm{n}}^{{\rm{2 + }}}}\) couple is \({\rm{ + 0}}{\rm{.15}}\,\,{\rm{V}}\) and that for the \({\rm{C}}{{\rm{r}}^{{\rm{3 + }}}}{\rm{/Cr}}\) couple is \({\rm{ - 0}}{\rm{.74}}\,\,{\rm{V}}\). These two couple in their standard state are connected to make a cell. The cell potential will be
330062
What will be the emf of the following concentration cell at \({\rm{25^\circ C}}\)?
\(\left. {{\rm{A}}{{\rm{g}}_{\left( {\rm{s}} \right)}}} \right \vert \left. {{\rm{AgN}}{{\rm{O}}_{\rm{3}}}{\rm{(0}}{\rm{.01M)}}} \right\vert \left. {{\rm{AgN}}{{\rm{O}}_{\rm{3}}}{\rm{(0}}{\rm{.05M)}}} \right \vert {\rm{A}}{{\rm{g}}_{{\rm{(s)}}}}\)
330064 Standard electrode potential for \({\rm{S}}{{\rm{n}}^{{\rm{4 + }}}}{\rm{/S}}{{\rm{n}}^{{\rm{2 + }}}}\) couple is \({\rm{ + 0}}{\rm{.15}}\,\,{\rm{V}}\) and that for the \({\rm{C}}{{\rm{r}}^{{\rm{3 + }}}}{\rm{/Cr}}\) couple is \({\rm{ - 0}}{\rm{.74}}\,\,{\rm{V}}\). These two couple in their standard state are connected to make a cell. The cell potential will be
330062
What will be the emf of the following concentration cell at \({\rm{25^\circ C}}\)?
\(\left. {{\rm{A}}{{\rm{g}}_{\left( {\rm{s}} \right)}}} \right \vert \left. {{\rm{AgN}}{{\rm{O}}_{\rm{3}}}{\rm{(0}}{\rm{.01M)}}} \right\vert \left. {{\rm{AgN}}{{\rm{O}}_{\rm{3}}}{\rm{(0}}{\rm{.05M)}}} \right \vert {\rm{A}}{{\rm{g}}_{{\rm{(s)}}}}\)
330064 Standard electrode potential for \({\rm{S}}{{\rm{n}}^{{\rm{4 + }}}}{\rm{/S}}{{\rm{n}}^{{\rm{2 + }}}}\) couple is \({\rm{ + 0}}{\rm{.15}}\,\,{\rm{V}}\) and that for the \({\rm{C}}{{\rm{r}}^{{\rm{3 + }}}}{\rm{/Cr}}\) couple is \({\rm{ - 0}}{\rm{.74}}\,\,{\rm{V}}\). These two couple in their standard state are connected to make a cell. The cell potential will be