319397
Solubility of four ionic salts \({{\rm{S}}_{\rm{1}}}{\rm{,}}\,\,{{\rm{S}}_{\rm{2}}}{\rm{,}}\,\,{{\rm{S}}_{\rm{3}}}\,\,{\rm{and}}\,\,{{\rm{S}}_{\rm{4}}}\) are plotted against temperature in Kelvin. Which of these salts will form exothermic solution \(\left( {{\rm{\Delta }}{{\rm{H}}_{{\rm{sol}}{\rm{.}}}}{\rm{ < 0}}} \right)\)?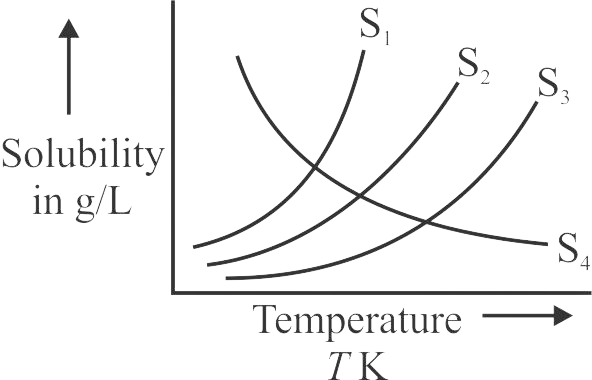
319397
Solubility of four ionic salts \({{\rm{S}}_{\rm{1}}}{\rm{,}}\,\,{{\rm{S}}_{\rm{2}}}{\rm{,}}\,\,{{\rm{S}}_{\rm{3}}}\,\,{\rm{and}}\,\,{{\rm{S}}_{\rm{4}}}\) are plotted against temperature in Kelvin. Which of these salts will form exothermic solution \(\left( {{\rm{\Delta }}{{\rm{H}}_{{\rm{sol}}{\rm{.}}}}{\rm{ < 0}}} \right)\)?
319397
Solubility of four ionic salts \({{\rm{S}}_{\rm{1}}}{\rm{,}}\,\,{{\rm{S}}_{\rm{2}}}{\rm{,}}\,\,{{\rm{S}}_{\rm{3}}}\,\,{\rm{and}}\,\,{{\rm{S}}_{\rm{4}}}\) are plotted against temperature in Kelvin. Which of these salts will form exothermic solution \(\left( {{\rm{\Delta }}{{\rm{H}}_{{\rm{sol}}{\rm{.}}}}{\rm{ < 0}}} \right)\)?
319397
Solubility of four ionic salts \({{\rm{S}}_{\rm{1}}}{\rm{,}}\,\,{{\rm{S}}_{\rm{2}}}{\rm{,}}\,\,{{\rm{S}}_{\rm{3}}}\,\,{\rm{and}}\,\,{{\rm{S}}_{\rm{4}}}\) are plotted against temperature in Kelvin. Which of these salts will form exothermic solution \(\left( {{\rm{\Delta }}{{\rm{H}}_{{\rm{sol}}{\rm{.}}}}{\rm{ < 0}}} \right)\)?
319397
Solubility of four ionic salts \({{\rm{S}}_{\rm{1}}}{\rm{,}}\,\,{{\rm{S}}_{\rm{2}}}{\rm{,}}\,\,{{\rm{S}}_{\rm{3}}}\,\,{\rm{and}}\,\,{{\rm{S}}_{\rm{4}}}\) are plotted against temperature in Kelvin. Which of these salts will form exothermic solution \(\left( {{\rm{\Delta }}{{\rm{H}}_{{\rm{sol}}{\rm{.}}}}{\rm{ < 0}}} \right)\)?