CHXII02:SOLUTIONS
319271
Two solutions marked as A and B are separated through semipermeable membrane as below. The phenomenon undergoing
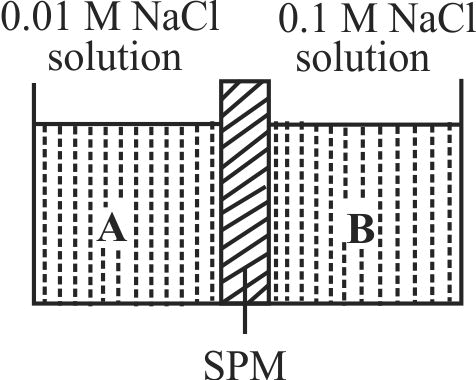
1 \({\rm{N}}{{\rm{a}}^{\rm{ + }}}\) moves from solution A to solution B
2 Both \({\rm{N}}{{\rm{a}}^{\rm{ + }}}{\mkern 1mu} {\mkern 1mu} {\rm{and}}{\mkern 1mu} {\mkern 1mu} {\rm{C}}{{\rm{l}}^{\rm{ - }}}\) moves from solution (1)
to solution (2)
3 Both \({\rm{N}}{{\rm{a}}^{\rm{ + }}}{\mkern 1mu} {\mkern 1mu} {\rm{and}}{\mkern 1mu} {\mkern 1mu} {\rm{C}}{{\rm{l}}^{\rm{ - }}}\) moves from solution (2) to (1)
4 Solvent molecules moves from solution (1) to (2)
Explanation:
During osmosis, only solvent molecules move from higher concentration region to lower concentration region.