319244
\({\mathrm{0.05 \mathrm{M} \,\mathrm{CuSO}_{4}}}\) when treated with \({\mathrm{0.01 \mathrm{M}\, \mathrm{K}_{2} \mathrm{Cr}_{2} \mathrm{O}_{7}}}\) gives green colour solution of \({\mathrm{\mathrm{Cu}_{2} \mathrm{Cr}_{2} \mathrm{O}_{7}}}\). The two solutions are separated as shown below: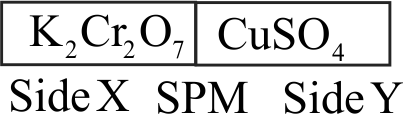
[SPM : Semi Permeable Membrane]
Due to osmosis
319245 Two solutions of \(\mathrm{KNO}_{3}\) and \(\mathrm{CH}_{3} \mathrm{COOH}\) are prepared separately. Molarity of both is 0.1 M and osmotic pressures are \({{\rm{P}}_{\rm{1}}}\) and \({{\rm{P}}_{\rm{2}}}\) respectively. The correct relationship between the osmotic pressures is
319244
\({\mathrm{0.05 \mathrm{M} \,\mathrm{CuSO}_{4}}}\) when treated with \({\mathrm{0.01 \mathrm{M}\, \mathrm{K}_{2} \mathrm{Cr}_{2} \mathrm{O}_{7}}}\) gives green colour solution of \({\mathrm{\mathrm{Cu}_{2} \mathrm{Cr}_{2} \mathrm{O}_{7}}}\). The two solutions are separated as shown below:
[SPM : Semi Permeable Membrane]
Due to osmosis
319245 Two solutions of \(\mathrm{KNO}_{3}\) and \(\mathrm{CH}_{3} \mathrm{COOH}\) are prepared separately. Molarity of both is 0.1 M and osmotic pressures are \({{\rm{P}}_{\rm{1}}}\) and \({{\rm{P}}_{\rm{2}}}\) respectively. The correct relationship between the osmotic pressures is
319244
\({\mathrm{0.05 \mathrm{M} \,\mathrm{CuSO}_{4}}}\) when treated with \({\mathrm{0.01 \mathrm{M}\, \mathrm{K}_{2} \mathrm{Cr}_{2} \mathrm{O}_{7}}}\) gives green colour solution of \({\mathrm{\mathrm{Cu}_{2} \mathrm{Cr}_{2} \mathrm{O}_{7}}}\). The two solutions are separated as shown below:
[SPM : Semi Permeable Membrane]
Due to osmosis
319245 Two solutions of \(\mathrm{KNO}_{3}\) and \(\mathrm{CH}_{3} \mathrm{COOH}\) are prepared separately. Molarity of both is 0.1 M and osmotic pressures are \({{\rm{P}}_{\rm{1}}}\) and \({{\rm{P}}_{\rm{2}}}\) respectively. The correct relationship between the osmotic pressures is
319244
\({\mathrm{0.05 \mathrm{M} \,\mathrm{CuSO}_{4}}}\) when treated with \({\mathrm{0.01 \mathrm{M}\, \mathrm{K}_{2} \mathrm{Cr}_{2} \mathrm{O}_{7}}}\) gives green colour solution of \({\mathrm{\mathrm{Cu}_{2} \mathrm{Cr}_{2} \mathrm{O}_{7}}}\). The two solutions are separated as shown below:
[SPM : Semi Permeable Membrane]
Due to osmosis
319245 Two solutions of \(\mathrm{KNO}_{3}\) and \(\mathrm{CH}_{3} \mathrm{COOH}\) are prepared separately. Molarity of both is 0.1 M and osmotic pressures are \({{\rm{P}}_{\rm{1}}}\) and \({{\rm{P}}_{\rm{2}}}\) respectively. The correct relationship between the osmotic pressures is
319244
\({\mathrm{0.05 \mathrm{M} \,\mathrm{CuSO}_{4}}}\) when treated with \({\mathrm{0.01 \mathrm{M}\, \mathrm{K}_{2} \mathrm{Cr}_{2} \mathrm{O}_{7}}}\) gives green colour solution of \({\mathrm{\mathrm{Cu}_{2} \mathrm{Cr}_{2} \mathrm{O}_{7}}}\). The two solutions are separated as shown below:
[SPM : Semi Permeable Membrane]
Due to osmosis
319245 Two solutions of \(\mathrm{KNO}_{3}\) and \(\mathrm{CH}_{3} \mathrm{COOH}\) are prepared separately. Molarity of both is 0.1 M and osmotic pressures are \({{\rm{P}}_{\rm{1}}}\) and \({{\rm{P}}_{\rm{2}}}\) respectively. The correct relationship between the osmotic pressures is