318182
\({\mathrm{\mathrm{CH} \equiv \mathrm{CH} \xrightarrow[\mathrm{NaOH}]{\mathrm{O}_{3}}(\mathrm{X}) \xrightarrow[\mathrm{CH}_{3} \mathrm{COOH}]{\mathrm{Zn}}}}\)
In the given reaction ethyne reacts with ozone in the presence of alkali forming X which further reacts with zinc in the presence of acetic acid forming Y . The ratio of molecular mass of X and Y is ____ .
318182
\({\mathrm{\mathrm{CH} \equiv \mathrm{CH} \xrightarrow[\mathrm{NaOH}]{\mathrm{O}_{3}}(\mathrm{X}) \xrightarrow[\mathrm{CH}_{3} \mathrm{COOH}]{\mathrm{Zn}}}}\)
In the given reaction ethyne reacts with ozone in the presence of alkali forming X which further reacts with zinc in the presence of acetic acid forming Y . The ratio of molecular mass of X and Y is ____ .
318182
\({\mathrm{\mathrm{CH} \equiv \mathrm{CH} \xrightarrow[\mathrm{NaOH}]{\mathrm{O}_{3}}(\mathrm{X}) \xrightarrow[\mathrm{CH}_{3} \mathrm{COOH}]{\mathrm{Zn}}}}\)
In the given reaction ethyne reacts with ozone in the presence of alkali forming X which further reacts with zinc in the presence of acetic acid forming Y . The ratio of molecular mass of X and Y is ____ .
318182
\({\mathrm{\mathrm{CH} \equiv \mathrm{CH} \xrightarrow[\mathrm{NaOH}]{\mathrm{O}_{3}}(\mathrm{X}) \xrightarrow[\mathrm{CH}_{3} \mathrm{COOH}]{\mathrm{Zn}}}}\)
In the given reaction ethyne reacts with ozone in the presence of alkali forming X which further reacts with zinc in the presence of acetic acid forming Y . The ratio of molecular mass of X and Y is ____ .
318182
\({\mathrm{\mathrm{CH} \equiv \mathrm{CH} \xrightarrow[\mathrm{NaOH}]{\mathrm{O}_{3}}(\mathrm{X}) \xrightarrow[\mathrm{CH}_{3} \mathrm{COOH}]{\mathrm{Zn}}}}\)
In the given reaction ethyne reacts with ozone in the presence of alkali forming X which further reacts with zinc in the presence of acetic acid forming Y . The ratio of molecular mass of X and Y is ____ .
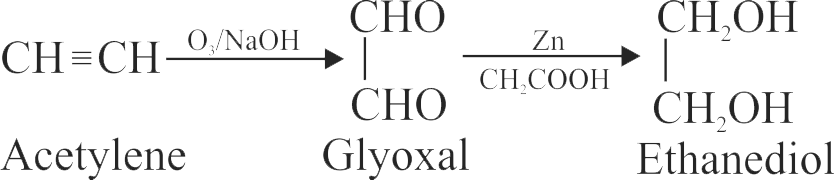
.png)
.png)
