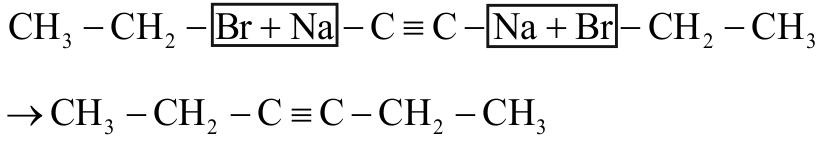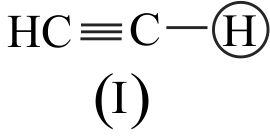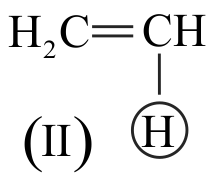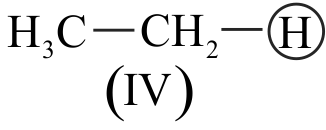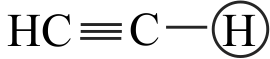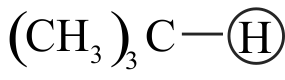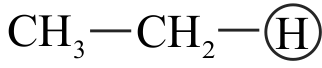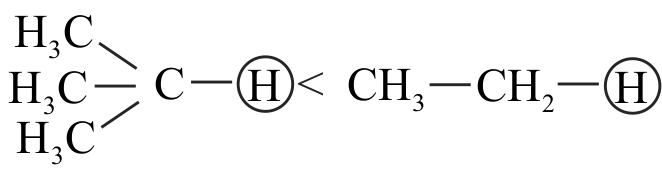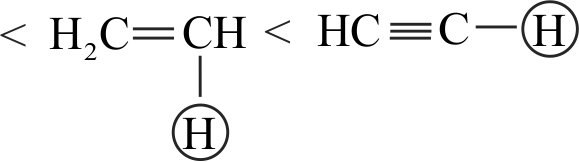318142
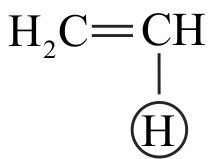
Which of the following order is correct regarding acidic character of hydrocarbons given below?
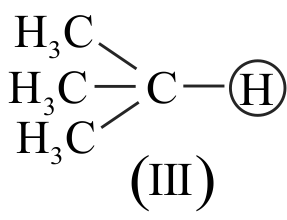
I. \(\mathrm{CH} \equiv \mathrm{CH}>\mathrm{CH}_{2}=\mathrm{CH}_{2}>\mathrm{CH}_{3}-\mathrm{CH}_{3}\)
II. \(\mathrm{HC} \equiv \mathrm{CH}>\mathrm{CH}_{3} \mathrm{C} \equiv \mathrm{CH}>\mathrm{CH}_{3} \mathrm{C} \equiv \mathrm{CCH}_{3}\)
318142
Which of the following order is correct regarding acidic character of hydrocarbons given below?
I. \(\mathrm{CH} \equiv \mathrm{CH}>\mathrm{CH}_{2}=\mathrm{CH}_{2}>\mathrm{CH}_{3}-\mathrm{CH}_{3}\)
II. \(\mathrm{HC} \equiv \mathrm{CH}>\mathrm{CH}_{3} \mathrm{C} \equiv \mathrm{CH}>\mathrm{CH}_{3} \mathrm{C} \equiv \mathrm{CCH}_{3}\)
318142
Which of the following order is correct regarding acidic character of hydrocarbons given below?
I. \(\mathrm{CH} \equiv \mathrm{CH}>\mathrm{CH}_{2}=\mathrm{CH}_{2}>\mathrm{CH}_{3}-\mathrm{CH}_{3}\)
II. \(\mathrm{HC} \equiv \mathrm{CH}>\mathrm{CH}_{3} \mathrm{C} \equiv \mathrm{CH}>\mathrm{CH}_{3} \mathrm{C} \equiv \mathrm{CCH}_{3}\)
318142
Which of the following order is correct regarding acidic character of hydrocarbons given below?
I. \(\mathrm{CH} \equiv \mathrm{CH}>\mathrm{CH}_{2}=\mathrm{CH}_{2}>\mathrm{CH}_{3}-\mathrm{CH}_{3}\)
II. \(\mathrm{HC} \equiv \mathrm{CH}>\mathrm{CH}_{3} \mathrm{C} \equiv \mathrm{CH}>\mathrm{CH}_{3} \mathrm{C} \equiv \mathrm{CCH}_{3}\)
318142
Which of the following order is correct regarding acidic character of hydrocarbons given below?
I. \(\mathrm{CH} \equiv \mathrm{CH}>\mathrm{CH}_{2}=\mathrm{CH}_{2}>\mathrm{CH}_{3}-\mathrm{CH}_{3}\)
II. \(\mathrm{HC} \equiv \mathrm{CH}>\mathrm{CH}_{3} \mathrm{C} \equiv \mathrm{CH}>\mathrm{CH}_{3} \mathrm{C} \equiv \mathrm{CCH}_{3}\)