318121
\(\mathrm{P}\) gives products \(\mathrm{Q}\) or \(\mathrm{R}\).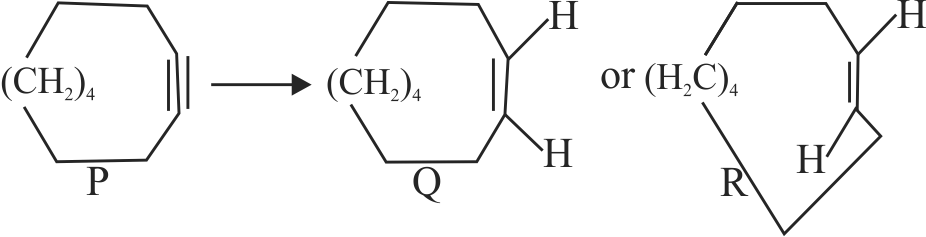
The possible reagents are:
(I) \({\text{2Na/liq}}{\text{.}}\,{\text{N}}{{\text{H}}_{\text{3}}}\)
(II) \({{\text{H}}_{\text{2}}}{\text{/Pd/C}}\,\,{\text{(quinoline)}}\)
The correct statement (s) with respect to the
above conversation is/are :
318121
\(\mathrm{P}\) gives products \(\mathrm{Q}\) or \(\mathrm{R}\).
The possible reagents are:
(I) \({\text{2Na/liq}}{\text{.}}\,{\text{N}}{{\text{H}}_{\text{3}}}\)
(II) \({{\text{H}}_{\text{2}}}{\text{/Pd/C}}\,\,{\text{(quinoline)}}\)
The correct statement (s) with respect to the
above conversation is/are :
318121
\(\mathrm{P}\) gives products \(\mathrm{Q}\) or \(\mathrm{R}\).
The possible reagents are:
(I) \({\text{2Na/liq}}{\text{.}}\,{\text{N}}{{\text{H}}_{\text{3}}}\)
(II) \({{\text{H}}_{\text{2}}}{\text{/Pd/C}}\,\,{\text{(quinoline)}}\)
The correct statement (s) with respect to the
above conversation is/are :
318121
\(\mathrm{P}\) gives products \(\mathrm{Q}\) or \(\mathrm{R}\).
The possible reagents are:
(I) \({\text{2Na/liq}}{\text{.}}\,{\text{N}}{{\text{H}}_{\text{3}}}\)
(II) \({{\text{H}}_{\text{2}}}{\text{/Pd/C}}\,\,{\text{(quinoline)}}\)
The correct statement (s) with respect to the
above conversation is/are :
318121
\(\mathrm{P}\) gives products \(\mathrm{Q}\) or \(\mathrm{R}\).
The possible reagents are:
(I) \({\text{2Na/liq}}{\text{.}}\,{\text{N}}{{\text{H}}_{\text{3}}}\)
(II) \({{\text{H}}_{\text{2}}}{\text{/Pd/C}}\,\,{\text{(quinoline)}}\)
The correct statement (s) with respect to the
above conversation is/are :
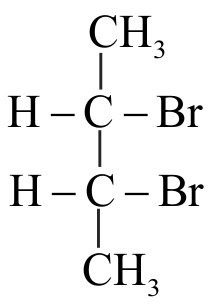
.png)
.png)
